Abstract
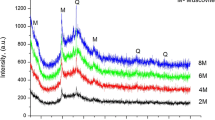
The present research explored the application of geopolymerization for the immobilization and solidification of heavy metal added into metakaolinte. The compressive strength of geopolymers was controlled by the dosage of heavy metal cations, and geopolymers have a toleration limit for heavy metals. The influence of alkaline activator dosage and type on the heavy metal ion immobilization efficiency of metakaolinte-based geopolymer was investigated. A geopolymer with the highest heavy metal immobilization efficiency was identified to occur at an intermediate Na2SiO3 dosage and the metal immobilization efficiency showed an orderly increase with the increasing Na+ dosage. Geopolymers with and without heavy metals were analyzed by the X-ray powder diffraction (XRD) and Fourier transform infrared (FTIR) spectroscopy. No crystalline phase containing heavy metals was detected in geopolymers with heavy metal, suggesting that the crystalline phase containing heavy metals is not produced or most of the phases incorporating heavy metals are amorphous. FTIR spectroscopy showed that, with increasing heavy metal addition, an increase in NO −3 peak intensity was observed, which was accompanied by a decrease in the CO 2−3 peak.
Similar content being viewed by others
References
Davidovits J. Geopolymers: inorganic polymeric new materials. Journal of Thermal Analysis, 1991, 37(8): 1633–1656
Kamel A Z, Mohammad S. Al-Harahsheh, Falah B H. Fly ash-based geopolymer for Pb removal from aqueous solution. Journal of Hazardous Materials, 2011, 188(1–3): 414–421
Yunsheng Z, Wei S, Qianli C, Lin C. Synthesis and heavy metal immobilization behaviors of slag based geopolymer. Journal of Hazardous Materials, 2007, 143(1–2): 206–213
van Deventer J S J, Provis J L, Duxson P, Lukey G C. Reaction mechanisms in the geopolymeric conversion of inorganic waste to useful products. Journal of Hazardous Materials, 2007, 139(3): 506–513
Zhang J, Provis J L, Feng D, van Deventer J S J. Geopolymers for immobilization of Cr6+, Cd2+, and Pb2+. Journal of Hazardous Materials, 2008, 157(2–3): 587–598
Phair J W, Van Deventer J S J. Effect of silicate activator pH on the leaching and material characteristics of waste-based inorganic polymers. Minerals Engineering, 2001, 14(3): 289–304
Bankowski P, Zou L, Hodges R. Using inorganic polymer to reduce leach rates of metals from brown coal fly ash. Minerals Engineering, 2004, 17(2): 159–166
van Jaarsveld J G S, Van Deventer J S J. Effect of the alkali metal activator on the properties of fly ash-based geopolymers. Industrial & Engineering Chemistry Research, 1999, 38(10): 3929–3941
Buchwald A, Zellmann H D, Kap Ch. Condensation of aluminosilicate gels-model system for geopolymer binders. Journal of Non-Crystalline Solids, 2011, 357(5): 1376–1382
Villa C, Pecina E T, Torres R, Gómez L. Geopolymer synthesis using alkaline activation of natural zeolite. Construction & Building Materials, 2010, 24(11): 2084–2090
Pandey B, Kinrade S D, Catalan L J J. Effects of carbonation on the leachability and compressive strength of cement-solidified and geopolymer-solidified synthetic metal wastes. Journal of Environmental Management, 2012, 101: 59–67
Xu J Z, Zhou Y L, Chang Q, Qu H Q. Study on the factors of affecting the immobilization of heavy metals in fly ash-based geopolymers. Materials Letters, 2006, 60(6): 820–822
Poon S, Lio K W. The limitation of the toxicity characteristic leaching procedure for evaluation cement-based stabilized/solidified waste forms. Waste Management (New York), 1997, 17(1): 15–23
Wang W, Zheng L, Wang F, Wan X, Yin K Q, Gao X B. Release of Elements from municipal solid waste incineration fly ash. Frontiers of Environmental Science & Engineering in China, 2010, 4(4): 482–489
van Jaarsveld J G S, van Deventer J S J, Lukey G C. The effect of composition and temperature on the properties of fly ash- and kaolinite-based geopolymers. Chemical Engineering Journal, 2002, 89(1–3): 63–73
Xiao Y, Lasaga A C. Ab initio quantum mechanical studies of the kinetics and mechanisms of silicate dissolution: H+ (H3O+) catalysis. Geochimica et Cosmochimica Acta, 1994, 58(24): 5379–5400
Zheng L, Wang W, Shi Y. The effects of alkaline dosage and Si/Al ratio on the immobilization of heavy metals in municipal solid waste incineration fly ash-based geopolymer. Chemosphere, 2010, 79(6): 665–671
Duxson P, Provis J L, Lukey G C, Mallicoat SW, Kriven WM, van Deventer J S J. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2005, 269(1–3): 47–58
Phair J W, van Deventer J S J. Effect of the silicate activator pH on the microstructura characteristics of waste-based geopolymers. International Journal of Mineral Processing, 2002, 66(1–4): 121–143
Zheng L, Wang C, Wang W, Shi Y, Gao X. Immobilization of MSWI fly ash through geopolymerization: effects of water-wash. Waste Management, 2011, 31: 311–317
Theo Kloprogge J, Wharton D, Hickey L, Frost R L. Infrared and raman study of interlayer anions CO 2−3 , NO −3 , 2−4 and ClO −4 in Mg/Al-hydrotalcite. American Mineralogist, 2002, 87: 623–629
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, L., Wang, W., Qiao, W. et al. Immobilization of Cu2+, Zn2+, Pb2+, and Cd2+ during geopolymerization. Front. Environ. Sci. Eng. 9, 642–648 (2015). https://doi.org/10.1007/s11783-014-0707-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11783-014-0707-4