Abstract
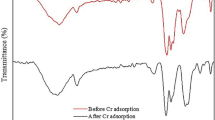
In this study, geopolymers were prepared using metakaolin (MK) raw material under different alkali activator moduli (SiO2/Na2O = 0.8, 1.2, 1.6, 2.0 M ratio) in order to analyze their capacity and mechanism for adsorbing cadmium (Cd2+) from solution. Instrumental analyses including X-ray diffraction (XRD), scanning electron microscopy (SEM) with energy dispersive spectroscopy (EDS), X-ray photoelectron spectroscopy(XPS), Fourier transform infrared (FTIR), and Brunauer-Emmett-Teller (BET) were performed to examine the mineralogical features of the MK and geopolymers before and after Cd2+ adsorption. The effect of initial pH, temperature, contact time, and initial concentration on Cd adsorption performance was studied to obtain the equilibrium isotherm. Kinetic data of the geopolymers fitted the pseudo-second-order kinetic model well. Moreover, the adsorption equilibrium data of Cd2+ adsorbed by the geopolymers fitted the Langmuir model better than the Freundlich model. The result shows that chemisorption dominates Cd2+ adsorption by geopolymers and that the adsorption capacity differs when prepared using different alkali-activated modulus agents. The geopolymer prepared using an alkali activator modulus of 0.8 M (molar ratio) exhibited the best Cd2+ adsorption performance with a maximum adsorption capacity of 70.3 mg g−1. The removal rate of Cd2+ by geopolymer still remained above 85% after five round of recycling.







Similar content being viewed by others
References
Almeida KA, Landers R, Cardoso D (2012) Properties of faujasite zeolites containing methyl-substituted ammonium cations. J Catal 294:151–160
Al-Zboon K, Al-Harahsheh MS, Hani FB (2011) Fly ash-based geopolymer for Pb removal from aqueous solution. J Hazard Mater 188:414–421
Andrejkovičová S, Sudagar A, Rocha J, Patinha C, Hajjaji W, da Silva EF, Velosa A, Rocha F (2016) The effect of natural zeolite on microstructure, mechanical and heavy metals adsorption properties of metakaolin based geopolymers. Appl Clay Sci 126:141–152
Barbosa TR, Foletto EL, Dotto GL, Jahn SL (2018) Preparation of mesoporous geopolymer using metakaolin and rice husk ash as synthesis precursors and its use as potential adsorbent to remove organic dye from aqueous solutions. Ceram Int 44:416–423
Bing W, Gao B, Wan Y (2017) Entrapment of ball-milled biochar in Ca-alginate beads for the removal of aqueous Cd2+. J Ind Eng Chem 61:161–168
Chao H, Zhu P, Min C, Hu H, Fu Q (2017) Comparative adsorption of Pb2+, Cu2+ and Cd2+ on chitosan saturated montmorillonite: kinetic, thermodynamic and equilibrium studies. Appl Clay Sci 143:320–326
Chen X, Niu Z, Wang J, Zhu GR, Zhou M (2018) Effect of sodium polyacrylate on mechanical properties and microstructure of metakaolin-based geopolymer with different SiO2/Al2O3 ratio. Ceram Int 44:18173–18180
Da̧browski A, Hubicki Z, Podkościelny P, Robens E (2004) Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere. 56:91–106
Deng J, Liu Y, Liu S, Zeng G, Tan X, Huang B, Tang X, Wang S, Hua Q, Yan Z (2017) Competitive adsorption of Pb2+, Cd2+ and Cu2+ onto chitosan-pyromellitic dianhydride modified biochar. J Colloid Interface Sci 506:355–364
Dermont G, Bergeron M, Mercier G, Richer-Laflèche M (2008) Soil washing for metal removal: a review of physical/chemical technologies and field applications. J Hazard Mater 152:1–31
Ding Z, Xin H, Wan Y, Wang S, Gao B (2016) Removal of lead, copper, cadmium, zinc, and nickel from aqueous solutions by alkali-modified biochar: batch and column tests. J Ind Eng Chem 33:239–245
Duan P, Yan C, Zhou W, Ren D (2016) Development of fly ash and iron ore tailing based porous geopolymer for removal of Cu2+ from wastewater. Ceram Int 42:13507–13518
Fan L, Lu Y, Yang L, Huang F, Ouyang X (2019) Fabrication of polyethylenimine-functionalized sodium alginate/cellulose nanocrystal/polyvinyl alcohol core-shell microspheres ((PVA/SA/CNC)@PEI) for diclofenac sodium adsorption. J Colloid Interface Sci 554:48–58
Heah CY, Kamarudin H, Bakri AMMA, Bnhussain M, Luqman M, Nizar IK, Ruzaidi CM, Liew YM (2012) Study on solids-to-liquid and alkaline activator ratios on kaolin-based geopolymers. Constr Build Mater 35:912–922
Holkar CR, Jadhav AJ, Pinjari DV, Mahamuni NM, Pandit AB (2016) A critical review on textile wastewater treatments: possible approaches. J Environ Manag 182:351–366
Huang J, Wu Z, Chen L, Sun Y (2015) Surface complexation modeling of adsorption of Cd2+ on graphene oxides. J Mol Liq 209(3):753–758
Kara İ, Yilmazer D, Akar ST (2017) Metakaolin based geopolymer as an effective adsorbent for adsorption of zinc2+ and nickel2+ ions from aqueous solutions. Appl Clay Sci 139:54–63
Kara I, Tunc D, Sayin F, Akar ST (2018) Study on the performance of metakaolin based geopolymer for Mn2+ and Co2+ removal. Appl Clay Sci 161:184–193
Karin Cristiane J, Fávere V, Laranjeira M, Ademir R, Peralta A (2005) Kinetics and equilibrium adsorption of Cu2+, Cd2+, and Ni2+ ions by chitosan functionalized with 2[−bis-(pyridylmethyl) aminomethyl]-4-methyl-6-formylphenol. J Colloid Interface Sci 291(2):369–374
Kim I, Rehman MSU, Han JI (2014) Fermentable sugar recovery and adsorption potential of enzymatically hydrolyzed rice straw. J Clean Prod 66:555–561
Kljajević LM, Nenadović SS, Nenadović MT, Bundaleski NK, Todorović BŽ, Pavlović VB, Rakočević ZL (2017) Structural and chemical properties of thermally treated geopolymer samples. Ceram Int 43:6700–6708
Li C, Zhang M, Zhong H, He H, Feng Y, Yin (2018) Synthesis of a bioadsorbent from jute cellulose, and application for aqueous Cd (II) removal. Carbohydrate Polymers 189:152–161
Liu Y, Chen W, Kim H (2013) Removal of lead and nickel ions from wastewater by genipin crosslinked chitosan/poly(ethylene glycol) Films. J Macromol Sci A 49(3):242–250
Liu Y, Yan C, Zhang Z, Wang H, Zhou S, Zhou W (2016) A comparative study on fly ash, geopolymer and faujasite block for Pb removal from aqueous solution. Fuel. 185:181–189
Luukkonen T, Sarkkinen M, Kemppainen K, Rämö J, Lassi U (2016) Metakaolin geopolymer characterization and application for ammonium removal from model solutions and landfill leachate. Appl Clay Sci 119:266–276
Madhava Rao M, Ramana DK, Seshaiah K, Wang MC, Chang Chien SW (2009) Removal of some metal ions by activated carbon prepared from Phaseolus aureus hulls. J Jazard Mater 166(2):1006–1013
Meng L, Shi W, Luo W, Hua X, Qiang G, Zhou C (2015) Facile synthesis and in situ magnetization of carbon-decorated lignocellulose fiber for highly efficient removal of methylene blue. J Chem Technol Biotechnol 90(6):1124–1134
Muhammad F, Huang X, Li S, Xia M, Zhang M, Liu Q, Shehzad Hassan MA, Jiao B, Yu L, Li D (2018) Strength evaluation by using polycarboxylate superplasticizer and solidification efficiency of Cr6+, Pb2+ and Cd2+ in composite based geopolymer. J Clean Prod 188:807–815
Mužek MN, Svilović S, Zelić J (2014) Fly ash-based geopolymeric adsorbent for copper ion removal from wastewater. Desalin Water Treat 52:2519–2526
Naghsh M, Shams K (2017) Synthesis of a kaolin-based geopolymer using a novel fusion method and its application in effective water softening. Appl Clay Sci 146:238–245
Ning C, Xu M, Hui DC-W, Lin CSK, McKay G (2017) Study of quench effect on heavy metal uptake efficiency by an aluminosilicate-based material. Chem Eng J 311:37–45
Panda L, Rath SS, Rao DS, Nayak BB, Das B, Misra PK (2018) Thorough understanding of the kinetics and mechanism of heavy metal adsorption onto a pyrophyllite mine waste based geopolymer. J Mol Liq 263:428–441
Qiu R, Cheng F, Huang H (2018) Removal of Cd2+ from aqueous solution using hydrothermally modified circulating fluidized bed fly ash resulting from coal gangue power plant. J Clean Prod 172:1918–1927
Ranjbar N, Mehrali M, Alengaram UJ, Metselaar HSC, Jumaat MZ (2014) Compressive strength and microstructural analysis of fly ash/palm oil fuel ash based geopolymer mortar under elevated temperatures. Constr Build Mater 65:114–121
Sarkar C, Basu JK, Samanta AN (2017) Removal of Ni2+ ion from waste water by geopolymeric adsorbent derived from LD Slag. J Water Proc Eng 17:237–244
Schneider P (1995) Adsorption isotherms of microporous-mesoporous solids revisited. Appl Catal A Gen 129:157–165
Shu Y, Wei X, Fang Y, Lan B, Chen H (2015) Removal of sulfuric acid mist from lead-acid battery plants by coal fly ash-based sorbents. J Hazard Mater 286:517–524
Singhal A, Gangwar BP, Gayathry JM (2017) CTAB modified large surface area nanoporous geopolymer with high adsorption capacity for copper ion removal. Appl Clay Sci 150:106–114
Siyal AA, Shamsuddin MR, Khan MI, Rabat NE, Zulfiqar M, Man Z, Siame J, Azizli KA (2018) A review on geopolymers as emerging materials for the adsorption of heavy metals and dyes. J Environ Manag 224:327–339
Sudagar A, Andrejkovičová S, Patinha C, Velosa A, Mcadam A, Silva EFD, Rocha F (2018) A novel study on the influence of cork waste residue on metakaolin-zeolite based geopolymers. Appl Clay Sci 152:196–210
Tan XF, Liu YG, Gu YL, Liu SB, Zeng GM, Cai X, Hu XJ, Wang H, Liu SM, Jiang LH (2016) Biochar pyrolyzed from MgAl-layered double hydroxides pre-coated ramie biomass (Boehmeria nivea (L.) Gaud.): characterization and application for crystal violet removal. J Environ Manag 184:85–93
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851
Wang RZ, Huang DL, Liu YG, Zhang C, Lai C, Zeng GM, Cheng M, Gong XM, Wan J, Luo H (2018) Investigating the adsorption behavior and the relative distribution of Cd2+ sorption mechanisms on biochars by different feedstock. Bioresour Technol 261
Wu W, Li J, Tian L, Müller K, Niazi NK, Xin C, Song X, Zheng L, Chu Y, Li J (2017) Unraveling sorption of lead in aqueous solutions by chemically modified biochar derived from coconut fiber: a microscopic and spectroscopic investigation. Sci Total Environ 576:766–774
Xu X, Cao X, Zhao L, Wang H, Yu H, Gao B (2013) Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environ Sci Pollut Res 20(1):358–368
Yan L, Xiao H, Liu Z, Meng M, Pan J, Jiang Y, Liang N, Wu W (2017) A novel dual temperature responsive mesoporous imprinted polymer for Cd2+ adsorption and temperature switchable controlled separation and regeneration. Chem Eng J 328:11–24
Yasemin K, Arpa, Cigdem, Tan S, Denizli A (2002) Biosorption of Hg2+ and Cd2+ from aqueous solutions: comparison of biosorptive capacity of alginate and immobilized live and heat inactivated Phanerochaete chrysosporium. Process Biochem 37(6):601–610
Zhang H, Omer AM, Hu Z, Yang LY, Ji C, Ouyang XK (2019) Fabrication of magnetic bentonite/carboxymethyl chitosan/sodium alginate hydrogel beads for Cu2+ adsorption. Int J Biol Macromol 135:490
Zhou W, Yan C, Duan P, Liu Y, Zhang Z, Qiu X, Li D (2016) A comparative study of high- and low-Al2O3 fly ash based-geopolymers: the role of mix proportion factors and curing temperature. Mater Des 95:63–74
Zibouche F, Kerdjoudj H, Damme HV (2009) Geopolymers from Algerian metakaolin. Influence of secondary minerals. Appl Clay Sci 43:453–458
Acknowledgments
We thank Tingyu Bai from the Spice and Beverage Research Institute, Chinese Academy of Tropical Agricultural Sciences, for the guidance on the operation of SEM-EDX.
Funding
This work was supported by the Project of the Ministry of Water Resources of China (Rapid evaluation of flight inspection of rural drinking water safety engineering projects).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lan, T., Li, P., Rehman, F.U. et al. Efficient adsorption of Cd2+ from aqueous solution using metakaolin geopolymers. Environ Sci Pollut Res 26, 33555–33567 (2019). https://doi.org/10.1007/s11356-019-06362-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06362-w