Abstract
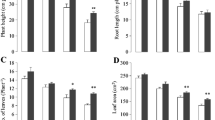
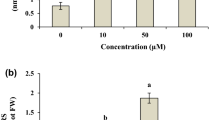
This study investigated the chromium (Cr) detoxification mechanism-induced changes in growth and antioxidant defence enzyme activities in Chrysopogon zizanioides. Plant growth decreased by 36.8% and 45.0% in the shoots and roots, respectively, in the 50 mg/L Cr treatment. Cr accumulation was higher in root (9807 μg/g DW) than in shoots (8730 μg/g DW). Photosynthetic pigments and malondialdehyde content increased up to the 30 mg/L Cr treatment, whereas they declined at higher doses. The activity of superoxide dismutase (SOD), catalase (CAT) and peroxidase (POX) were increased significantly with increasing of Cr dose but slightly declined at higher doses. Isozyme banding patterns revealed the expression of multiple bands for SOD, CAT and POX enzymes, and the band intensity decreased at high doses of Cr exposure. These results indicate that higher Cr doses increased the oxidative stress by over production of reactive oxygen species (ROS) in vetiver that had potential tolerance mechanism to Cr as evidenced by enhanced level of antioxidative enzymes, photosynthetic pigments, MDA contents. Therefore, vetiver has evolved a mechanism for detoxification and accumulates higher concentration of toxic Cr. This study provides a better understanding of how vetiver detoxifies Cr.
摘要
本文研究了铬(Cr)对香根草(Chrysopogon zizanioides)生长以及抗氧化防御酶活性的影响, 探讨其对Cr 的解毒机制。在50 mg/L Cr 处理条件下,植物地上部分和地下部分的生长量分别降低了 36.8%和45.0%;Cr 在植物地下部分的积累量(9807 μg/g 干重)大于地上部分的积累量(8730 μg/g 干重)。随着Cr 添加量的增加,光合色素和丙二醛含量增加,在30 mg/L Cr 处理条件下含量达到最高, 之后,随着Cr 处理浓度的增加略有下降;同时,抗氧化防御酶(超氧化物歧化酶、过氧化氢酶和过 氧化物酶)的活性显著提高,在较高浓度Cr 处理下略有下降。同工酶结果显示在高浓度Cr 处理下, 超氧化物歧化酶、过氧化氢酶和过氧化物酶的条带强度降低。目前研究结果表明,高浓度Cr 导致活 性氧过量产生,从而增加了对香根草的氧化损伤,而光合色素、丙二醛、抗氧化酶含量的增加表明香 根草对Cr 有潜在的耐性机制。结果表明,香根草具有对Cr 的解毒机制,并可以积累高浓度的Cr。本 文为更深入地研究根草对Cr 的解毒机制提供了依据。
Similar content being viewed by others
References
CHEN T L, WISE S S, KRAUS S, SHAFFIEY F, LEVINE K, THOMPSON D W, ROMANO T, HARA T, WISE J P. Particulate hexavalent chromium is cytotoxic and genotoxic to the North Atlantic right whale (Eubalaena glacialis) lung and skin fibroblasts [J]. Environmental and Molecular Mutagenesis, 2009, 50: 387–393. DOI: 10.1002/em.2047.
SHANKER A K, CERVANTES C, LOZATAVERAC H, AVUDAINAYAGAM S. Chromium toxicity in plants [J]. Environment International, 2005, 31: 739–753.
DUBE B K, TEWARI K, CHATTERJEE J, CHATTERJEE C. Excess chromium alters uptake and translocation of certain nutrients in Citrullus [J]. Chemosphere, 2003, 53: 1147–1153. DOI: 10.1016/S0045-6535(03)00570-8.
WANG Jun, CHENG Qing, XUE Sheng-guo, RAJENDRAN M, WU Chuan, LIAO Jia. Pollution characteristics of surface runoff under different restoration types in manganese tailing wasteland. [J. Environmental Science and Pollution Research, 2018, 10: 9998–10005. DOI: 10.1007/s11356-018-1338-2.
XUE Sheng-guo, YE Yu, ZHU Feng, WANG Qiong, JIANG Jun, HARTLEY W. Changes in distribution and microstructure of bauxite residue aggregates following amendments addition [J]. Journal of Environmental Sciences, 2019, 78: 276–286. DOI: 10.1016/j.jes.2018.10.010.
ZHANG A, FENG H, YANG G. Unventilated indoor coal-fired stoves in Guizhou province, China: Cellular and genetic damage in villagers exposed to arsenic in food and air [J]. Environmental Health Perspectives, 2007, 115: 653–658. DOI: 10.1289/ehp.9272.
WU Chuan, SHI Li, XUE Sheng-guo, LI Wai-chin, JIANG Xing-xing, RAJENDRAN M, QIAN Zi. Effect of sulfur-iron modified biochar on the available cadmium and bacterial community structure in contaminated soils [J]. Science of the Total Environment, 2019, 647: 1158–1168. DOI: 10.1016/j.scitotenv.2018.08. 087.
GASPAR T, PENEL C, HAGEGE D, GREPPIN H. Peroxidases in plant growth: Differentiation and development processes [C]//LOBARZEWSKI J, GREPPIN H, PENEL C, GASPAR T. Biochemical, Molecular and Physiological Aspects of Plant Peroxidases. Lublin, 1991: 249–280 Lublin.
XUE Sheng-guo, WU Yu, LI Yi-wei, KONG Xiang-feng, ZHU Feng, HARTLEY W, LI Xiao-fei, YE Yu. Industrial wastes applications for alkalinity regulation in bauxite residue: A comprehensive review [J]. Journal of Central South University, 2019, 26(2): 268–288.
ZOU Qi, AN Wen, WU Chuan, LI Wai-chin, FU An-qing, XIAO Rui-yang, CHEN Hui-kang, XUE Sheng-guo. Red mud modified biochar reduces soil arsenic availability and changes bacterial composition [J]. Environmental Chemistry Letters, 2018, 16(3): 615–622. DOI: 10.1007/s10311-017-0688–1.
XUE Sheng-guo, LI Meng, JIANG Jun, MILLAR G J, LI Chu-xuan, KONG Xiang. Phosphogypsum stabilization of bauxite residue: Conversion of its alkaline characteristics [J]. Journal of Environmental Sciences, 2019, 77: 1–10. DOI: 10.1016/j.jes.2018.05.016.
SINGH V, THAKUR L, MONDAL P. Removal of lead and chromium from synthetic wastewater using Vetiveria zizanioides [J]. Clean Soil Air Water, 2015, 43–44: 538–543. DOI: 10.1002/clen.201300578.
MANIKANDAN R, EZHILI N, VENKATACHALAM P. Phosphorus supplementation alleviates the cadmium induced toxicity by modulating oxidative stress mechanisms in vetiver grass (Chrysopogon zizanioides) [J]. Journal of Environmental Engineering, 2016. DOI: 10.1061/(ASCE)EE. 1943–7870.0001112.
LIU W, YANG Y S, LI P J, ZHOU Q X, XIL L J, HAN X P. Risk assessment of cadmium contaminated soil on plant DNA damage using RAPD and physiological indices [J]. Journal of Hazards Materials, 2009, 161: 878–823. DOI: 10.1016/j.jhazmat.2008.04.038.
AL-SAADI S A A M, AL-SAADI W M, AL-WAHEEB A N H, The effect of some heavy metals accumulation on physiological and anatomical characteristic of some Potamogeton L. plant [J]. Journal of Ecology and Environmental Science, 2013, 4: 100–108. DOI: 10.1134/S1995082917020031.
ARNON D I. Copper enzymes in isolated chloroplasts: polyphenol oxidase in Beta valgaris [J]. Plant Physiology, 1949, 24: 1–15. DOI: 10.1104/pp.24.1.1.
DAS M, MAITI S K. Metal accumulation in five native plants growing on abandoned cu-tailings ponds [J]. Applies Ecology Environmental Research, 2007, 5: 27–35. DOI: 10.15666/aeer/0501_027035.
DAUD K, MEI L, VARIATH M T, ALI S, LI C, RAFIQ M T, ZHU S J. Chromium (VI) uptake and tolerance potential in cotton cultivars: Effect on their root physiology, ultramorphology, and oxidative metabolism [J]. Biomedical Research International, 2014: Article ID 975946. DOI: 10.1155/2014/975946.
DHINDSA R A, PLUMB D P, THORPE T A. Leaf senescence: Correlated with increased permeability and lipid peroxidation and decreased levels of superoxide dismutase and catalase [J]. Journal of Experimental Botany, 1981, 126: 93–101. DOI: 10.1093/jxb/32.1.93.
AEBI H. Catalase in vitro [J]. Methods in Enzymology, 1984, 105: 121–126. DOI: 10.1016/S0076-6879(84)05016-3.
CASTILLO F I, PENEL I, GREPPIN H. Peroxidase release induced by ozone in Redum album leaves [J]. Plant Physiology, 1984, 74: 846–851.
BEAUCHAMP C, FRIDOVICH I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels [J]. Analysis Biochemistry, 1971, 44: 276–287. DOI: 10.1016/0003-2697(71)90370-8.
VERMA S, DUBEY R S. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants [J]. Plant Science, 2003, 164: 645–655. DOI: 10.1016/s0168-9452(03)00022-0.
ANDERSON D, PRASAD K, STEWART R. Changes in isozyme Profiles of catalase, peroxidase and Glutathione reductase during acclimation to chilling in mesocotyls of maize seedlings [J]. Plant Physiology, 1995, 109: 1247–1257. DOI: 10.2307/4276926.
BRADFORD M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding [J]. Analysis of Biochemistry, 1976, 44: 276–287. DOI: 10.1016/0003-2697(76)90527-3.
DAVENPORT S B, GALLEGO S M, BENAVIDES M P, TOMAROW M L. Behaviour of antioxidant defense system in the adaptive response to salt stress in Helianthus annus L [J]. Plant Cell Growth Regulator, 2003, 40: 81–88. DOI: 10.1023/A: 1023060211546.
MICHAEL P I, KRISHNASWAMY M. The effect of zinc stress combined with high irradiance stress on membrane damage and antioxidative response in bean seedlings [J]. Environmental and Experimental Botany, 2011, 74: 171–177. DOI: 10.1016/j.envexpbot.2011.05.016.
SERGIEV I, ALXIEVA V, KARANOV E. Effect of sperm one, atrazine and combination between them on some endogenous protective systems and stress markers in plants [J]. Comptes Rendus de Academie Bulgare des Sciences, 1997, 51: 121–124. DOI: 10.1016/j.envexpbot.2011.05.016.
MALLICK S, SINAM G, MISHRA RK, SINHA S. Interactive effects of Cr and Fe treatments on plants growth, nutrition and oxidative status in Zea mays L [J]. Ecotoxicology and Environmental Safety, 2010, 73: 987–995. DOI: 10.1016/j.ecoenv.2010.03.004.
SHAHIDA M, SHAMSHAD S, RAFIQ M, KHALID S, BIBI I, NIAZI NK, DUMAT C, RASHID M I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review [J]. Chemosphere, 2017, 178: 513–533. DOI: 10.1016/j.chemosphere.2017.03.074.
RASHAD M B, MUHAMMAD A S, CHRISTOPHER V, LICOLN Z, GUODONG L, NEIL S M, BALA R, JUAN J M N, FRANCISCO G S. Kinnow mandarin plants grafted on tetraploid roots tocks are more tolerant to Cr-toxicity than those grafted on its diploids one [J]. Environmental and Experimental Botany, 2017, 140: 8–18. DOI: 10.1016/j.envexpbot.2017.05.011.
SEN A K, MONDAL N G, MONDAL S. Studies of uptake and toxic effects of Cr (VI) on Pisita stratioites [J]. Water Science and Technology, 1987, 19: 119–127. DOI: 10.1007/978-90-481-2666-8_53.
SIEGEL H. Metal ion in biological systems [M]. New York: Marcel Dekker, 1973: 2.
SINHA V, PAKSHIRAJAN K, CHATURVEDI R. Chromium (VI) accumulation and tolerance by Tradescantia pallid: Biochemical and antioxidant study [J]. Applied Biochemistry and Biotechnology, 2014, 173: 2297–2306. DOI: 10.1007/s12010-014-1035-7.
SAMARDAKIEWICZ S, WOZNY A. The distribution of lead in duckweed (Lemna minor L.) root tip [J]. Plant Soil, 2000, 226: 107–111. DOI: 10.1023/a: 1026440730839.
SINHA S, SAXENA R, SINGH S. Chromium induced lipid peroxidation in the plants of Pistia stratiotes L.: Role of antioxidants and antioxidant enzymes [J]. Chemosphere, 2005, 58: 595–604. DOI: 10.1016/j.chemosphere.2004.08. 071.
PANDEY V, DIXIT V, SHYAM R. Chromium effect on ROS generation and detoxification in pea (Pisum sativum) leaf chloroplasts [J]. Protoplasma, 2009, 236: 85–95. DOI: 10.1007/s00709-009-0061-8.
DHIR B, SHARMILA P, PARDHA SARADHI P, NASIM S A. Physiological and antioxidant responses of Salvinia natans exposed to chromium-rich waste water [J]. Ecotoxicology and Environmental Safety, 2009, 72: 1790–1797. DOI: 10.1016/j.ecoenv.2009.03.015.
ELSTNER E F. Oxygen activation and oxygen toxicity [J]. Annual Review of Plant Physiology, 1983, 33: 73–96. DOI: 10.1146/annurev.pp.33.060182.000445.
MILONE M T, SGHERRI C, CLIJSTERS H, NAVARI IZZO F. Antioxidative responses of wheat treated with realistic concentration of cadmium [J]. Environmental and Experimental Botany, 2003, 50: 265–276. DOI: 10.1016/S0098-8472(03)00037-6.
SUPARNA P, RITA K. Study of metal resistance potential of the Cd, Cr tolerant Alligator Weed [J]. Journal of Stress Physiology and Biochemistry, 2014, 10: 1244–1261.
PLATA S J, ORTEGA VILLASANTE C, FLORES CACERES M L, ESCOBAR C, DEL CAMPO F F, HERNANDEZ L E. Differential alterations of antioxidant defenses as bioindicators of mercury and cadmium toxicity in Alfalfa [J]. Chemosphere, 2009, 77: 946–954. DOI: 10.1016/j.chemosphere.2009.08.007.
MALAR S, MANIKANDAN R, PAULO J C F, SAHI S V, VENKATACHALAM P. Effect of lead on phytotoxicity, growth, biochemical alterations and its role on genomic template stability in Sesbania grandiflora: A potential plant for phytoremediation [J]. Ecotoxicology and Environmental Safety, 2014, 108: 249–257. DOI: 10.1016/j.ecoenv. 2014.05.018.
YU Xiao, LIN Yu, FAN Wei, LU Ming. The role of exogenous proline in amelioration of lipid peroxidation in rice seedlings exposed to Cr (VI) [J]. International Biodeterioration and Biodegradation, 2017, 123: 106–112. DOI: 10.1016/j.ibiod.2017.06.010.
LIU Chun, CHAO Yun, KAO Ching. Abscisic acid is an inducer of hydrogen peroxide production in leaves of rice seedlings grown under potassium deficiency [J]. Botany Studies, 2012, 53: 229–237. DOI: 10.1007/s12600-012-0219-3.
DAT J F, VANDENBEELE S, VRANOVA E, van MONTAGU M, INZE D, van BREUSEGEM F. Dual action of the active oxygen species during plant stress responses [J]. Cellular and Molecular Life Science, 2000, 57: 779–795. DOI: 10.1007/s000180050041.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Project(41771512) supported by the National Natural Science Foundation of China; Project(2018RS3004) supported by Hunan Science &Technology Innovation Program, China
Rights and permissions
About this article
Cite this article
Rajendran, M., An, Wh., Li, Wc. et al. Chromium detoxification mechanism induced growth and antioxidant responses in vetiver (Chrysopogon zizanioides(L.) Roberty). J. Cent. South Univ. 26, 489–500 (2019). https://doi.org/10.1007/s11771-019-4021-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-019-4021-y