Abstract
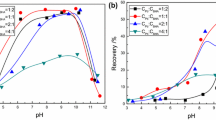
Flotation behavior and adsorption mechanism of octyl hydroxamic acid (OHA) on wolframite were investigated through flotation experiments, adsorption tests, zeta-potential measurements, infrared spectroscopy and solution chemistry calculations. Results of flotation and adsorption experiments show that the maximum values of flotation recovery and adsorption capacity occur around pH 9. In term of the solution chemistry calculations, the concentration of metal hydroxamate is greater than that of metal tungstate and metal hydroxyl, and metal hydroxamate compounds are identified to be the main species on wolframite surface at pH region of 8-10, contributing to the increase of OHA adsorption and flotation performance. Results of zeta-potential and IR spectra demonstrate that OHA adsorbs onto wolframite surface by chemisorptions. Hydroxamate ions can bond with Mn2+/Fe2+ cations of wolframite surface, forming metal hydroxamate compounds, which is a key factor in inducing the hydrophobicity of wolframite under the conditions of maximum flotation.
Similar content being viewed by others
References
FU Guang-qin, HE Xiao-juan, ZHOU Xiao-tong. The research progress of wolframite slime flotation [J]. China Tungsten Industry, 2010, 25(1): 22–25. (in Chinese)
SRIVASTAVA J P, PATHAK P N. Pre-concentration: A necessary step for upgrading tungsten ore [J]. International Journal of Mineral Processing, 2000, 60(1): 1–8.
WEI Da-wei, WEI Ke-wu, QIU Ji-cun. Hydrophobic agglomeration and spherical agglomeration of wolframite fines [J]. International Journal of Mineral Processing, 1986, 17(3/4): 261–271.
SRINIVAS K, SREENIVAS T, PADMANABHAN N P H, VENUGOPAL R. Studies on the application of alkyl phosphoric acid ester in the flotation of wolframite [J]. Mineral Processing and Extractive Metallurgy Review: An International Journal, 2004, 25(4): 253–267.
LIU De-quan, ZHOU Jun-shan, WANG Dian-zuo. Flotation interaction of wolframite with benzylarsonic acid and sodium butyl xanthate [J]. The Chinese Journal of Nonferrous Metals, 1992, 2(3): 25–29. (in Chinese)
MARABINIA A M, CIRIACHIB M, PLESCIAC P, BARBAROB M. Chelating reagents for flotation [J]. Minerals Engineering, 2007, 20(10): 1014–1025.
PRADIP. Applications of chelating agents in mineral processing [J]. Minerals and Metallurgical Processing, 1988, 5: 80–89.
QIN Wen-qing, REN Liu-yi, XU Yang-bao, WANG Pei-pei, MA Xi-hong. Adsorption mechanism of mixed salicyhydroxamic acid and tributyl phosphate collectors in fine cassiterite electro-flotation system [J]. Journal of Central South University, 2012, 19(6): 1711–1717.
ADAM J, CHRISTOPHER M, OLGA K, KRISTIAN E. Surface chemistry considerations in the flotation of bastnäsite [J]. Minerals Engineering, 2014, 66–68: 119–129.
REN Jun, WANG Wen-mei, LUO Jia-ke, ZHOU Gao-yun, TANG Fang-qiong. Progress of flotation reagents of rare earth minerals in China [J]. Journal of Rare Earths, 2003, 21(1): 1–8.
LIU Jin-wei, HU Hui-ping, WANG Meng, CHEN Xiang-pan, CHEN Qi-yuan, DING Zhi-ying. Synthesis of modified polyacrylamide with high content of hydroxamate groups and settling performance of red mud [J]. Journal of Central South University, 2015, 22: 2073–2080.
SREENIVAS T, PADMANABHAN N P H. Surface chemistry and flotation of cassiterite with alkyl hydroxamates [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2002, 205(1/2): 47–59.
PRADIP P, FUERSTENAU D W. Design and development of novel flotation reagents for the beneficiation of mountain pass rare-earth ore [J]. Minerals and Metallurgical Processing, 2013, 30(1): 1–9.
BASILIO C, LOWE R A, GORKEN A, MAGLIOCCO L, HAGY R. Modified hydroxamate collectors for kaolin flotation [J]. Developments in Mineral Processing, 2000, 13: C8b-51-C8b-55.
HU Yue-hua, WANG Dian-zuo, XU Zheng-he. A study of interaction and flotation of wolframite with octyl hydroxamate [J]. Minerals Engineering, 1997, 10(6): 623–633.
YANG Si-yuan, FENG Qi-ming, QIU Xian-yang, GAO Yu-de, XIE Zhen-fu. Relationship between flotation and Fe/Mn ratio of wolftamite with benzohydroxamic acid and sodium oleate as collectors [J]. Physicochemical Problems of Mineral Processing, 2014, 50(2): 747–758.
GIBSON C E, KELEBEK S, AGHAMITIAN M, YU B. Flotation of pyrochlore from low grade carbonatite gravity tailings with benzohydroxamic acid [J]. Minerals Engineering, 2015, 71: 97–104.
XIAO Ni, QI Liu. The adsorption and configuration of octyl hydroxamic acid on pyrochlore and calcite [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2012, 411: 80–86.
WANG Pei-pei, QIN Wen-qing, REN Liu-yi, WEI Qian, LIU Rui-zeng, YANG Cong-ren, ZHONG Shui-ping. Solution chemistry and utilization of alkyl hydroxamic acid in flotation of fine cassiterite [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(6): 1789–1796.
FUERSTENAU D W, PRADI P. Mineral flotation with hydroxamate collectors [M]// Reagents in the Minerals Industry. London: IMM, 1984: 161–168.
YE Zhi-ping. Study on the flotation mechanism of wolframite with benzohydroxamic acid [J]. Nonferrous Metals, 2000(5): 35–39. (in Chinese)
SREENIVAS T, PADMANABHAN N P H. Surface chemistry and flotation of cassiterite with alkyl hydroxamates [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2002, 205(1/2): 47–59.
SMITH R M, MARTELL A E. Critical stability constants: Inorganic complexes [M]. New York: Plenum Press, 1976: 5–19.
MARINAKI K I, KELSALL G H. Adsorption of decyl sulphate and decyl phosphate on wolframite, (Fe,Mn)WO4, and their use in the two-liquid flotation of fine wolframite particles [J]. Journal of Colloid and Interface Science, 1985, 106(2): 517–531.
HOPE G A, WOODS R, PARKER G K, BUCKLEY A N, MCLEAN J. A vibrational spectroscopy and XPS investigation of the interaction of hydroxamate reagents on copper oxide minerals [J]. Minerals Engineering, 2010, 23(11/12/13): 952–959.
CUI J, HOPE G A, BUCKLEY A N. Spectroscopic investigation of the interaction of hydroxamate with bastnaesite (cerium) and rare earth oxides [J]. Minerals Engineering, 2012, 36-38: 91–99.
MOENKE H. Mineral spektren [M]. Berlin: Akademie-Verlag, 1966: 33.
LU Yong-quan, DENG Zhen-hua. Analysis of practical infrared spectrum [M]. Beijing: Electronic Industry Press, 1989: 132. (in Chinese)
Author information
Authors and Affiliations
Additional information
Foundation item: Project(2014CB643402) supported by the National Basic Research Program of China; Project(CX2013B082) supported by the Hunan Provincial Innovation Foundation for Postgraduate, China
Rights and permissions
About this article
Cite this article
Meng, Qy., Feng, Qm. & Ou, Lm. Flotation behavior and adsorption mechanism of fine wolframite with octyl hydroxamic acid. J. Cent. South Univ. 23, 1339–1344 (2016). https://doi.org/10.1007/s11771-016-3185-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-016-3185-y