Abstract
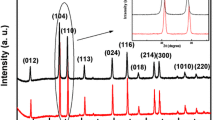
Well-crystallized hexagonal hematite (α-Fe2O3) platelets were synthesized by hydrothermal process, using a highly concentrated ferric hydroxide as precursor. The precursor was prepared by adding ammonia to the ferric sulfate solution which was obtained by leaching pyrite cinders with sulfuric acid. Structure and morphology of the synthesized products were investigated by X-ray diffraction, scanning electron microscope, transmission electron microscope and selected area electron diffraction. The results reveal that the reaction temperature has significant effects on the structure, size and shape of the synthesized hematite particles. Typical hexagonal hematite platelets, about 0.4–0.6 μm in diameter and 0.1 μm in thickness, were prepared at 230 °C for 0.5 h. Al3+, contained in the sulfuric acid leaching solution as an impurity, plays an extremely important role in the formation of hexagonal hematite. In addition, a possible mechanism about the formation of hexagonal hematite platelets was proposed.
Similar content being viewed by others
References
CORNELL R M, SCHWERTMANN U. The iron oxide structure, properties, reactions, occurrences, and uses [M]. Weinhein: Wiley-VCH, 2003: 130–137.
SARKAR A, SETH D, DOZIER A K, NEATHERY J K, HAMDEH H H, DAVIS B H. Fischer-Tropsch synthesis: Morphology, phase transformation and particle size growth of nano-scale particles [J]. Catal Lett, 2007, 117(1/2): 1–17.
CHEN Jun, XU Li-na, LI Wen-yang, GOU Xing-long. α-Fe2O3 nanotubes in gas sensor and lithium-ion battery applications [J]. Adv Mater, 2005, 17(5): 582–586.
ZHONG Liang-shu, HU Jin-song, LIANG Han-pu, CAO An-min, SONG Wei-guo, WAN Li-jun. Self-assembled 3D flowerlike iron oxide nanostructures and their application in water treatment [J]. Adv Mater, 2006, 18(18): 2426–2431.
NULI Y, ZENG R, ZHANG P, GUO Z P, LIU H K. Controlled synthesis of α-Fe2O3 nanostructures and their size-dependent electrochemical properties for lithium-ion batteries [J]. J Power Sources, 2008, 184(2): 456–461.
TADIC M, MARKOVIC D, SPASOJEVIC V, KUSIGERSKI V, REMSKAR M, PIRNAT J, JAGLICIC Z. Synthesis and magnetic properties of concentrated α-Fe2O3 nanoparticles in a silica matrix [J]. J Alloys Compd, 2007, 441(1/2): 291–296.
WANG Dan-jun, LI Dong-sheng, GUO Li, FU Feng, QI Guang-cai, WANG Yao-yu. Synthesis and characterization of spherical hematite (α-Fe2O3) nanopowder by sonochemical hydrolysis method [J]. Chinese J Inorg Chem, 2006, 22(7): 1317–1320.
LU Jian, CHEN Dai-rong, XIU Jiao-ling. Fabrication, characterization, and formation mechanism of hollow spindle-like hematite via a solvothermal process [J]. J Colloid Interface Sci, 2006, 303(2): 437–443.
QIU Jin, YANG Ru, LI Min, JIANG Nan. Preparation and characterization of porous ultrafine Fe2O3 particles [J]. Mater Res Bull, 2005, 40(11): 1968–1975.
JIA Chun-jiang, SUN Ling-dong, YAN Zheng-guang, YOU Li-ping, LUO Feng, HAN Xiao-dong, PANG Yu-cheng, ZHANG Ze, YAN Chun-hua. Single-crystalline iron oxide nanotubes [J]. Angew Chem Int Edit, 2005, 44(28): 4328–4333.
SUBER L, IMPERATORI P, AUSANIO G, FABBRI F, HOFMEISTER H, Synthesis, morphology, and magnetic characterization of iron oxide nanowires and nanotubes [J]. J Phys Chem B, 2005, 109(15): 7103–7109.
WU Chang-zheng, YIN Ping, ZHU Xi, OUYANG Chuan-zi, XIE Yi. Synthesis of hematite (α-Fe2O3) nanorods: Diameter-size and shape effects on their applications in magnetism, lithium ion battery, and gas sensors [J]. J Phys Chem B, 2006, 110(36): 17806–17812.
HOU Bo, WU You-shi, WU Li-li, SHI Yuan-chang, ZOU Ke, GAI Hong-de. Hydrothermal synthesis of cubic ferric oxide particles [J]. Mater Lett, 2006, 60(25/26): 3188–3191.
SUGIMOTO T, WAKI S, ITOH H, MURAMATSU A. Preparation of monodisperse platelet-type hematite particles from a highly condensed β-FeOOH suspension [J]. Colloids and Surfaces A, 1996, 109(11): 155–165.
SUGIMOTO T, WANG Y. Mechanism of the shape and structure control of monodispersed α-Fe2O3 particles by sulfate ions [J]. J Colloid Interface Sci, 1998, 207(1): 137–149.
KANDORI K, YAMAMOTO N, YASUKAWA A, ISHIKAWA T. Preparation and characterization of disk-shaped hematite particles by a forced hydrolysis reaction in the presence of polyvinyl alcohol [J]. Phys Chem Chem Phys, 2002, 4(1): 6116–6122.
DIAMANDESCU L, D. MIHAILA-TARABASANU D, POPECSU-POGRION N, TOTOVINA A, BIBICU I. Hydrothermal synthesis and characterization of some polycrystalline α-iron oxides [J]. Ceram Int, 1999, 25(8): 689–692.
GIUNTI M, BARONI D, BACCI E. Hazard assessment to workers of trace metal content in pyrite cinders [J]. Bull Environ Contam Toxicol, 2004, 72(2): 352–357.
TUGRUL N, DERUN E M, PISKIN M. Utilization of pyrite ash wastes by pelletization process [J]. Powder Technol, 2007, 176(2): 72–76.
AIP I, DEVECI H, YAZICI E Y, TURK T, SUNGUN Y H. Potential use of pyrite cinders as raw material in cement production: Results of industrial scale trial operations [J]. J Hazard Mater, 2009, 166(1): 144–149.
ZHENG Ya-jie, CHEN Bai-zhen, GONG Zhu-qing, CHEN Wen-mi. A new method of preparing polymeric ferric sulfate from pyrite cinders [J]. Journal of Central South University: Science and Technology, 2001, 32(2): 142–145. (in Chinese)
NAVROTSKY A, MAZEINA L, MAJZLAN J. Size-driven structural and thermodynamic complexity in iron oxides [J]. Science, 2008, 319(5870): 1635–1638.
CAO Fu-Ling, WU Yu-Fei, LIU Hui, WEI Yu. Preparation and property of discoid aluminum-doped α-Fe2O3 particles [J]. Acta Chimica Sinica, 2008, 66(12): 1405–1410.
SCHWERTMANN U, FITZPATRICK R W, TAYLOR R M, LEWIS D G. The influence of aluminum on the iron oxides: Part II. Preparation and properties of Al-substituted hematites [J]. Clays Clay Miner, 1979, 27(2): 105–112.
LIU Hui, WEI Yu, SUN Yu-han. The formation of hematite from ferrihydrite using Fe(II) as a catalyst [J]. J Mol Catal A Chem, 2005, 226(1): 135–140.
JIA Bao-ping, GAO Lian, Sun Jing. Synthesis of single crystalline hematite polyhedral nanorods via a facile hydrothermal process [J]. J Am Ceram Soc, 2007, 90(4): 1315–1318.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Project(2008A090300016) supported by the Key Science and Technology Item of Guangdong Province, China; Project(ZKJ2010022) supported by the Precious Apparatus Opening Center Foundation of Central South University, China
Rights and permissions
About this article
Cite this article
Liu, Zc., Zheng, Yj. Hexagonal hematite platelets synthesized from pyrite cinders by hydrothermal process. J. Cent. South Univ. Technol. 18, 1377–1382 (2011). https://doi.org/10.1007/s11771-011-0849-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-011-0849-5