Abstract
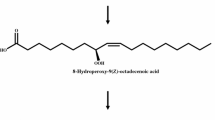
A previously established method was utilized to determine the stereoconfiguration of 7,10-dihydroxy-8(E)-octadecenoic acid (DHOE) from bioconversion of oleic acid by Pseudomonas aeruginosa NRRL strain B-18602 (PR3). The method involved formation of the (−)-menthoxycarbonyl (MCO) derivative of the two hydroxyls, oxidative cleavage of the double bond, and gas chromatography (GC) analysis of the two methyl-esterified diastereomeric fragments, methyl 2-MCO-decanoate and dimethyl 2-MCO-octanedioate. As described by previous workers, the 2(S)-MCO derivatives elute at earlier times by GC than the 2(R)-MCO derivatives. By comparing the GC analysis of the 2-MCO derivatives obtained from DHOE with that obtained from a partially racemized sample, DHOE was determined to be 7(S),10(S)-dihydroxy-8(E)-octadecenoic acid.
Similar content being viewed by others
References
Hou, C.T., M.O. Bagby, R.D. Plattner, and S. Koritala, A Novel Compound, 7,10-Dihydroxy-8(E)-octadecenoic Acid from Oleic Acid by Bioconversion, J. Am. Oil Chem. Soc. 68:99–101 (1991).
Hou, C.T., and M.O. Bagby, Production of a New Compound, 7,10-Dihydroxy-8(E)-octadecenoic Acid from Oleic Acid by Pseudomonas sp. PR3, J. Indust. Microbiol. 7:123–130 (1991).
Hou, C.T., and M.O. Bagby, 10-Hydroxy-8(Z)-octadecenoic Acid, an Intermediate in the Bioconversion of Oleic Acid to 7,10-Dihydroxy-8(E)-octadecenoic Acid, Ibid.:103–107 (1992).
Knothe, G., M.O. Bagby, R.E. Peterson, and C.T. Hou, 7,10-Dihydroxy-8(E)-octadecenoic Acid: Stereochemistry and a Novel Derivative, 7,10-Dihydroxyoctadecenoic Acid, J. Am. Oil Chem. Soc. 69:367–371 (1992).
Hou, C.T., L.K. Nakamura, D. Weisleder, R.E. Peterson, and M.O. Bagby, Identification of NRRL Strain B-18602 (PR3) as Pseudomonas aeruginosa and Effect of Phenazine 1-Carboxylic Acid Formation on 7,10-Dihydroxy-8(E)-octadecenoic Acid Accumulation, World J. Microbiol. Biotechnol. 9:570–573 (1993).
Parra, J.L., J. Pastor, F. Comelles, M.A. Manresa, and M.P. Bosch, Studies of Biosurfactants Obtained from Olive Oil, Tenside Surf. Det. 27:302–306 (1990).
Mercade, E., M. Robert, M.J. Espuny, M.P. Bosch, M.A. Manresa, J.L. Parra, and J. Guinea, New Surfactant Isolated from Pseudomonas 42A2, J. Am. Oil Chem. Soc. 65:1915–1916 (1988).
Guerrero, A., I. Casals, M. Busquets, Y. Leon, and A. Manresa, Oxidation of Oleic Acid to (E)-10-Hydroperoxy-8-octadecenoic and (E)-10-Hydroxy-8-octadecenoic Acids by Pseudomonas sp. 42A2, Biochim. Biophys. Acta 1347:75–81 (1997).
De Andrés, C., E. Mercadé, J. Guinea, and A. Manresa, 7,10-Dihydroxy-8(E)-octadecenoic Acid Produced by Pseudomonas 42A2: Evaluation of Different Cultural Parameters of the Fermentation, World J. Microbiol. Biotechnol. 10:106–109 (1994).
Hamberg, M., Steric Analysis of Hydroperoxides Formed by Lipoxygenase Oxygenation of Linoleic Acid, Anal. Biochem. 43:515–526 (1971).
Hamberg, M., R.P. Herman, and U. Jacobsson, Stereochemistry of Two Epoxy Alcohols from Saprolegnia parasitica, Biochim. Biophys. Acta 879:410–418 (1986).
Westley, J.W., and B. Halpern, The Use of (−)-Menthyl Chloroformate in the Optical Analysis of Asymmetric Amino and Hydroxyl Compounds by GAs Chromatography, J. Org. Chem. 33:3978–3980 (1968).
Hammarström, S., Configuration of 2-Hydroxy Acids from Brain Cerebrosides Determined by Gas Chromatography, FEBS Lett. 5:192–195 (1969).
Fahlstadius, P., and M. Hamberg, A Gas-Liquid Chromatographic Method for Steric Analysis of Epoxy Acids, Chem. Phys. Lipids 51:15–22 (1989).
Gardner, H.W., T.D. Simpson, and M. Hamberg, Transformation of Fatty Acid Hydroperoxides by Alkali and Characterization of Products, Lipids 28:487–495 (1993).
Oliw, E.H., H. Sprecher, and M. Hamberg, Isolation of Two Novel E Prostaglandins in Human Seminal Fluid, J. Biol. Chem. 261:2675–2683 (1986).
Brodowsky, I.D., and E.H. Oliw, Metabolism of 18:2(n-6), 18:3(n-3), 20:4(n-6) and 20:5(n-3) by the Fungus Gaeumannomyces graminis: Identification of Metabolites Formed by 8-Hydroxylation and by ω2 and ω3 Oxygenation, Biochim. Biophys. Acta 1124:59–65 (1992).
Matthew, J.A., H.W.-S. Chan, and T. Galliard, A Simple Method for the Preparation of Pure 9-d-Hydroperoxide of Linoleic Acid and Methyl Linoleate Based on the Positional Specificity of Lipoxygenase in Tomato Fruit, Lipids 12:324–326 (1977).
Chan, H.W.-S., and G. Levett, Autoxidation of Methyl Linoleate. Separation and Analysis of Isomeric Mixtures of Methyl Linoleate Hydroperoxides and Methyl Hydroxylinoleates, Ibid.:99–104 (1977).
Brash, A.R., and D.J. Hawkins, High-Performance Liquid Chromatography for Chiral Analysis of Eicosanoids, Meth. Enzymol. 187:187–195 (1990).
Knothe, G., M.O. Bagby, D. Weisleder, and R.E. Peterson, Allylic Mono- and Dihydroxylation of Isolated Double Bonds with Selenium Dioxide-tert-Butyl Hydroperoxide. NMR Characterization of Long-Chain Enols, Allylic and Saturated 1,4-Diols, and Enones, J. Chem. Soc., Perkin 2:1661–1669 (1994).
Powell, R.G., C.R. Smith Jr., and I.A. Wolff, Geometric Configuration and Etherification Reactions of Some Naturally Occurring 9-Hydroxy-10,12-and 13-Hydroxy-9,11-octadecadienoic Acids, J. Org. Chem. 32:1442–1446 (1967).
Mills, J.A., Correlations Between Monocyclic and Polycyclic Unsaturated Compounds from Molecular Rotation Differences, J. Chem. Soc.:4976–4985 (1952).
Scott, A.I., and A.D. Wrixon, Stereochemistry of Olefins—IX Correlation of Mills’ and Brewster’s Rules with the Cotton Effects of Cyclic Olefins, Tetrahedron 27:4787–4819 (1971).
Beecham, A.F., The Influence of Allylic Oxygen on the π → π* CD of Certain Chromophores, Ibid.:5207–5216 (1971).
Smith, Jr., C.R., Optically Active Long-Chain Compounds and Their Absolute Configurations, in Topics in Lipid Chemistry, edited by F.D. Gunstone, Logos Press, London, 1970, Vol. 1, pp. 277–368.
Phillips, B.E., C.R. Smith, Jr., and L.W. Tjarks, (S)-13-Hydroxy-cis-9,trans-11-octadecadienoic Acid Lactone, a 14-Membered-Ring Compound from Monnina emarginata Seed Oil, J. Org. Chem. 35:1916–1919 (1970).
Koshino, H., S. Togiya, T. Yoshihara, S. Sakamura, T. Shimanuki, T. Sato, and A. Tajimi, Four Fungitoxic C-18 Hydroxy Unsaturated Fatty Acids from Stromata of Epichloe typhina, Tetrahedron Lett. 28:73–76 (1987).
Martin, V.S., and K.B. Sharpless, General Method for Determining Absolute Configuration of Acyclic Allylic Alcohols, J. Am. Chem. Soc. 104:3775–3776 (1982).
Schneider, C., P. Schreier, and H.-U. Humpf, Exciton-Coupled Circular Dichroism (ECCD) in Acyclic Hydroxylated Dienes: A Sensitive Method for the Direct Stereochemical Assignment of Lipoxygenase Products, Chirality 9:563–567 (1997).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Gardner, H.W., Hou, C.T. All (S) stereoconfiguration of 7,10-dihydroxy-8(E)-octadecenoic acid from bioconversion of oleic acid by Pseudomonas aeruginosa . J Am Oil Chem Soc 76, 1151–1156 (1999). https://doi.org/10.1007/s11746-999-0088-1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11746-999-0088-1