Abstract
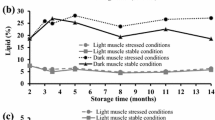
Light muscle, dark muscle, and skin from herring (Clupea harengus) were stored separately or as intact fillets at −18°C. After 0, 2, 8, 12, and 18 wk, all tissues were analyzed for conjugated dienes (A234) and lipid hydroperoxides. In tissues stored separately, total absorbance at 268 nm (A268) and lipid-soluble fluorescent oxidation products (FP) were also monitored. Further, prior to storage these tissues were subjected to measurement of total lipids, lipid classes, fatty acid pattern, α-tocopherol, iron, copper, selenium, and total aqueous pro-oxidative activity. When light muscle, dark muscle, and skin were stored as intact fillets, the following ranking order was seen for A234 and levels of lipid hydroperoxides at the end of the storage period: skin > dark muscle > light muscle. The corresponding ranking order for tissues stored separately was: dark muscle > skin > light muscle, whereas for A268 and FP the orders were: dark muscle > light muscle > skin and light muscle > dark muscle > skin, respectively. The compositional data obtained indicate the highest level of pro-oxidants in dark muscle and the highest level of polar lipids in light muscle. These observations reveal that pro-oxidants, to a greater extent than lipid composition, influence the increase in A234, hydroperoxides, and A268, whereas the reverse seems to be true for the increase in FP. The results also point to the strong influence from oxygen contact and tissue interactions on the progress of lipid oxidation in herring during storage.
Similar content being viewed by others
References
Hultin, H.O., Oxidation of Lipids in Seafoods, in Seafoods Chemistry, Processing Technology and Quality, edited by F. Shahidi and J.R. Botta, Blackie A&P, London, 1994, pp. 47–74.
Eriksson, C.E., Lipid Oxidation Catalysts and Inhibitors in Raw Materials and Processed Foods, Food Chem. 9:3–9 (1981).
Vicetti, R., and J. Palma, Oxidation Susceptibility of Lipids in Whole Frozen Sardines Stored at −5°, Boletin de Investigacion Instituto Pesquero 2:45–56 (1984).
Ke, P.J., R.G. Ackman, B.A. Linke, and D.M. Nash, Differential Lipid Oxidation in Various Parts of Frozen Mackerel, J. Food Technol. 12:37–47 (1977).
Toyomizu, M., and K. Hanoka, Lipid Oxidation of the Minced Ordinary Muscle of Fish During Storage at −5°C and Susceptibility to Lipid Oxidation, Bull. Jap. Soc. Sci. Fish 46:1007–1010 (1980).
Yamaguchi, K., T. Nakamura, and M. Toyomizu, Preferential Lipid Oxidation in the Skin of Round Fish. Ibid:5869–5874 (1984).
Hultin, H.O., Biochemical Deterioration of Fish Muscle, in Quality Assurance in the Fish Industry, edited by H.H. Huss, M. Jakobsen, and J. Liston, Elsevier, Amsterdam, 1992, pp. 125–138.
German, J.B., and J.E. Kinsella, Lipid Oxidation in Fish Tissue. Enzymatic Initiation via Lipoxygenase, J. Agric. Food Chem. 33:680–683 (1985).
Slabyj, B.M., and H.O. Hultin, Lipid Peroxidation by Microsomal Fractions Isolated from Light and Dark Muscle of Herring (Clupea harengus), J. Food Sci. 47:1395–1398 (1982).
Slabyj, B.M., and H.O. Hultin, Microsomal Lipid Peroxidation Systems from Herring Light and Dark Muscle: Effect of Cytosolic Fractions, J. Food Biochem. 7:107–114 (1983).
Petillo, D., and H.O. Hultin, Antioxidant Loss in Atlantic Mackerel (Scomber scombrus) Light and Dark Muscle, in Institute of Food Technologists Annual Meeting, 1995, p. 180.
Jia, T-D., S.D. Kelleher, H.O. Hultin, D. Petillo, R. Maney, and J. Krzynowek, Comparison of Quality Loss and Changes in the Glutathioneperoxidase Antioxidant System in Stored Mackerel and Bluefish Muscle, J. Agric. Food Chem. 44:1195–1201 (1996).
Erickson, M.C., Compositional Parameters and Their Relationship to Oxidative Stability of Channel Catfish, Ibid.:1213–1218 (1993).
Hultin, H.O., Lipid Oxidation in Fish Muscle, in Advances In Seafood Biochemistry, edited by G.J. Flick, and R.E. Martin, Technomic, Lancaster, 1992, pp. 99–122.
Tichivangana, J.Z., and P.A. Morrisey, Lipid Oxidation in Cooked Fish Muscle, Ir. J. Food Sci. Technol. 6:157–163 (1982).
Mai, J., and J.E. Kinsella, Lipid Composition of Dark and White Muscle from White Sucker (Catostomus commersoni), J. Food Sci. 44:1101–1109 (1979).
Ke, P.J., and R.G. Ackman, Metal-Catalyzed Oxidation in Mackerel Skin and Meat Lipids, J. Am. Oil Chem. Soc. 53:636–640 (1976).
Mohri, S., S.-Y. Cho, Y. Endo, and K. Fujimoto, Lipoxygenase Activity in Sardine Skin, Agric. Biol. Chem. 54:1889–1891 (1990).
Undeland, I., M. Stading, and H. Lingnert, Influence of Skinning on Lipid Oxidation in Different Horizontal Layers of Herring (Clupea harengus) During Frozen Storage, J. Sci. Food Agr., in press.
Ingemansson, T., P. Kaufmann, and A. Pettersson, Lipid Hydrolysis and Oxidation Related to Astaxanthin Content in Light and Dark Muscle of Frozen Stored Rainbow Trout (Oncorhynchus mykiss), J. Food Sci. 58:513–518 (1993).
Isaak, R.A., and W.C. Johnsson, Collaborative Study of Wet and Dry Ashing Techniques for the Elemental Analysis of Plant Tissue by Atomic Absorption Spectrophotometry, J. Assoc. Off. Anal. Chem. 58:436–440 (1975).
Standard Conditions for Iron, in Perkin-Elmer Cookbook, Vol. 2, Perkin-Elmer, Norwalk, 1976.
The THGA Graphite Furnace: Techniques and Recommended Conditions, Perkin-Elmer Publication B3210, Bodenseewerk Perkin-Elmer GmbH, Ueberlingen, Germany, 1992, 16 pp.
Flow Injection Hydride Analyses: Recommended Analytical Conditions and General Information, Perkin-Elmer Publication B3505, Bodenseewerk Perkin-Elmer GmbH, Ueberlingen, Germany, 1994, pp. 22–23.
Axelrod, B., T.M. Cheesbrough, and S. Laakso, in Methods in Enzymology, edited by J.M. Lowenstein, Academic Press, London, 1981, vol. 71, pp. 441–451.
Rutgersson, A., E.-L. Bergman, H. Lingnert, and A.-S. Sandberg, Optimization of Temperature, Time and Lactic Acid Concentration to Inactivate Lipoxygenase and Lipase and Preserve Phytase Activity in Barley (cv. Blenheim) During Soaking, Cereal Chem. 74:727–732 (1997).
Bligh, E.G., and W.J. Dyer, A Rapid Method of Total Lipid Extraction and Purification, Can. J. Biochem. Physiol. 37:911–917 (1959).
Ekstrand, B., I. Gangby, G. Åkesson, U. Stöllman, H. Lingnert, and S. Dahl, Lipase Activity and Development of Rancidity in Oats and Oat Products Related to Heat Treatment During Processing, J. Cereal Sci. 17:247–254 (1993).
Burton, G.W., A. Webb, and K.U. Ingold, A Mild, Rapid, Efficient Method of Lipid Extraction for Use in Determining Vitamin E/Lipid Ratios, Lipids 20:29–39 (1985).
Undeland, I., M. Härröd, and H. Lingnert, Comparison Between Methods Using Low Toxicity Solvents for the Extraction of Lipids from Herring (Clupea harengus), Food Chem. 61:355–365 (1998).
Kaluzny, M.A., L.A. Duncan, M.V. Merrit, and D.E. Epps, Rapid Separation of Lipid Classes in High Yield and Purity Using Bonded Phase Columns, J. Lipid Res. 26:135–140 (1985).
Piironen, V., P. Naro, E-L. Syväoja, K. Salminen, and P. Koivistoinen, High-Performance Liquid Chromatographic Determination of Tocopherols and Tocotrienols and Its Application to Diets and Plasma of Finnish Men, Int. Vit. Nutr. Res. 53:35–40 (1983).
Miyazawa, T., R. Saeki, and H. Inaba, Detection of Chemiluminescence in Lipid Peroxidation of Biological Systems and Its Application to HPLC, J. Biolumin. Chemilumin. 4:475–478 (1989).
Yamamoto, Y., and B.N. Ames, Detection of Lipid Hydroperoxides and Hydrogen Peroxide at Picomole Levels by an HPLC and Isoluminol Chemiluminescence Assay, Free Radical Biol. Med. 3:359–361 (1987).
Ruzicka, J., and E.H. Hanssen, Principles, in Flow Injection Analysis, edited by P.J. Elvin and J.D. Winefordner, John Wiley & Sons, 1981, pp. 6–29.
Undeland, I., and H. Lingnert, Measurement of Oxidative Changes in Fatty Fish Using UV- and Chemiluminescence Detection, Proceedings from the 18th Nordic Lipid Symposium, Reykjavik, Iceland, edited by G.G. Haraldsson, S. Gudbjarnason, and G. Lambertsen, 1995, pp. 139–143.
Miyazawa, T., T. Suzuki, K. Fujimoto, and K. Yasuda, Chemiluminescent Simultaneous Determination of Phosphatidylcholine Hydroperoxide and Phosphatidylethanolamine Hydroperoxide in the Liver and Brain of the Rat, J. Lipid Res. 33:1051–1059 (1992).
Anhydrous Milk Fat: Determination of Peroxide Value, Int. IDF Standard 74A, International Dairy Federation, Brussels, Belgium (1991).
Ueda, S., T. Hayashi, and M. Nakimi, Effect of Ascorbic Acid on Lipid Autoxidation in a Model Food System, Agric. Biol. Chem. 50:1–7 (1986).
Paquot, C., Evidence of Purity and Determination from Ultraviolet Spectrophotometry, IUPAC Standard Method II.D.23, in Standard Methods for the Analysis of Oils, Fats and Derivatives, 6th edn., A. Wheaton & Co. Ltd., Exeter, Great Britain, 1979.
Brown, H.G., and H.E. Snyder, Conjugated Dienes of Crude Soy Oil: Detection by UV Spectrophotometry and Separation by HPLC, J. Am. Oil Chem. Soc. 59:280–283 (1982).
Parr, L.J., and P.A.T. Swoboda, The Assay of Conjugable Oxidation Products Applied to Lipid Deterioration in Stored Foods, J. Food Technol. 11:12 (1976).
Dillard, C.J., and A.L. Tappel, Fluorescent Products from Reaction of Peroxidizing Polyunsaturated Fatty Acids with Phosphatidyl Ethanolamine and Phenylalanine, Lipids 8:183–189 (1973).
Love, R.M., Chemistry and Anatomy, in The Chemical Biology of Fishes, Academic Press, London, 1970, pp. 1–35.
Love, R.M., The Physical Structure of Fish Muscle and Its Chemistry, in The Food Fishes, Farrand Press, London, 1988, pp. 3–23.
Decker, E.A., and H.O. Hultin, Factors Influencing Catalysis of Lipid Oxidation by the Soluble Fraction of Mackerel Muscle, J. Food Sci. 55:947–950 (1990).
Decker, E.A., C-H. Huang, J.E. Osinchak, and H.O. Hultin, Iron and Copper: Role in Enzymic Lipid Oxidation of Fish Sarcoplasmic Reticulum at in situ Concentrations, J. Food Biochem. 13:179–186 (1989).
Flohé, L., Glutathione Peroxidase Brought into Focus, in Free Radicals in Biology, edited by W.E.M. Pryor, Academic Press, New York, 1982, pp. 223–253.
Kadiiska, M.B., P.M. Hanna, S.J. Jordan, and R.P. Mason, Electron Spin Resonance Evidence for Free Radical Generation in Copper-Treated Vitamin E- and Selenium-Deficient Rats: In Vivo Spin Trapping Investigation, Molecular Pharmacol. 44:222–227 (1993).
Nakano, T., M. Sato, and M. Takeuchi, Glutathione Peroxidase of Fish, J. Food Sci. 57:1116–1119 (1992).
Watanabe, F., M. Goto, K. Abe, and Y. Nakano, Glutathione Peroxidase Activity During Storage of Fish Muscle, Ibid.:734–735 (1996).
Stadtman, T.C., Biosynthesis and Function of Selenocysteine-Containing Enzymes, J. Biol. Chem. 266:16257–16260 (1991).
Nagayama, F., S. Imano, and Y. Naito, Effect of Temperature on Lipid Oxidation Catalyzed by Mackerel Tissue, Bull. Jpn. Soc. Sci. Fish 32:415–418 (1971).
Cho, S-Y., Y. Endo, K. Fujimoto, and T. Kaneda, Autoxidation of Ethyl Eicosapentaenoate in a Defatted Fish Dry Model System, Nippon Suisan Gakkaishi 53:545–552 (1989).
Fujimoto, K., S. Mohri, K. Hasegawa, and Y. Endo, Oxidative Deterioration of Fish Meat, Food Rev. Int. 6:603–616 (1990).
Eriksson, C.E., P.A. Olsson, and S.G. Svensson, Denaturated Hemoproteins as Catalysts in Lipid Oxidation, J. Am. Oil Chem. Soc. 48:442–447 (1971).
Eriksson, C.E., P.A. Olsson, and S.G. Svensson, Oxidation of Fatty Acids by Heat Treated Hemoproteins, Lipids 5:365–366 (1970).
Bohdan, M., M.B. Slabyj, and H.O. Hultin, Lipid Peroxidation by Microsomal Fractions Isolated from Light and Dark Muscles of Herring (Clupea harengus), J. Food Sci. 47:1395–1398 (1982).
Undeland, I., B. Ekstrand, and H. Lingnert, Lipid Oxidation in Minced Herring (Clupea harengus) During Frozen Storage. Effect of Washing and Precooking, J. Agric. Food Chem., in press.
Wang, Y-J., L.A. Miller, and P.B. Addis, Effect of Heat Inactivation of Lipoxygenase on Lipid Oxidation in Lake Herring (Coregonus artredii), J. Am. Oil Chem. Soc. 68:752–757 (1991).
Hägg, M., and J. Kumpulainen, E-Vitaminhalten hos Fisk, Poster No D3, Livsmedelsdagarna, October 26–27th, SIK-The Swedish Institute for Food and Biotechnology, Göteborg, Sweden (1994).
Situnayake, R.D., B.J. Crump, A.V. Zezulka, M. Davis, B. McConkey, and D.I. Thurnham, Measurement of Conjugated Diene Lipids by Derivative Spectroscopy in Heptane Extracts of Plasma, Ann. Clin. Biochem. 27:258–266 (1990).
Mizushima, Y., K. Takama, and K. Zama, Effect of Copper, Iron, and Hemin on Lipid Oxidation in Fish Flesh Homogenate, Bull. Fac. Fish Hokkaido Univ. 28:207–211 (1977).
Harris, P., and J. Tall, in Rancidity in Foods, edited by J.C. Allen and R.J. Hamilton, Blackie A&P, London, 1989, pp. 256–273.
Gutteridge, M.C., R. Richmond, and B. Halliwell, Inhibition of the Iron-Catalysed Formation of Hydroxyl Radicals from Superoxide and of Lipid Peroxidation by Desferrioxamine, Biochem. J. 184:469–472 (1979).
Kanner, J., and S. Harel, Initiation of Lipid Peroxidation by Activated Metmyoglobin and Methemoglobin, Arch. Biochem. Biophys. 237:314–321 (1985).
Hultin, H.O., Potential Lipid Oxidation Problems in Fatty Fish Processing, in Fatty Fish Utilization: Upgrading from Feed to Food, edited by N. Davis, University of North Carolina Sea Grant College Program, Raleigh, 1988, pp. 185–223.
Beckman, J.K., S.M. Borowitz, H.L. Green, and I.M. Burr, Promotion of Iron-Induced Rat Liver Microsomal Lipid Peroxidation by Copper, Lipids 23:559–563 (1988).
Kanner, J., I. Shegalovich, S. Harel, and B. Hazan, Muscle Lipid Peroxidation Dependent on Oxygen and Free Metal Ions, J. Agric. Food Chem. 36:409–412 (1988).
Gray, J.I., Measurement of Lipid Oxidation: A Review, J. Am. Oil Chem. Soc. 55:539–546 (1978).
Fletcher, B.L., C.J. Dillard, and L. Tappel, Measurement of Fluorescent Lipid Peroxidation Products in Biological Systems and Tissues, Anal. Biochem. 52:1–9 (1973).
Pokorny, J., B.A. El-Zeany, and G. Janicek, Browning Reactions of Oxidized Fish Lipids with Proteins, IV Int. Congress Food Sci. Technol. 1:217–223 (1974).
Hasegawa, K., Y. Endo, and K. Fujimoto, Oxidative Deterioration in Dried Fish Model Systems Assessed by Solid Sample Fluorescence Spectrophotometry, J. Food Sci. 57:1123–1126 (1992).
Gardner, C.W., Reactions of Hydroperoxides—Products of High Molecular Weight, in Autoxidation of Unsaturated Lipids, edited by H.W.S. Chan, Academic Press, London, 1987, pp. 51–93.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Undeland, I., Ekstrand, B. & Lingnert, H. Lipid oxidation in herring (Clupea harengus) light muscle, dark muscle, and skin, stored separately or as intact fillets. J Amer Oil Chem Soc 75, 581–590 (1998). https://doi.org/10.1007/s11746-998-0069-9
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11746-998-0069-9