Abstract
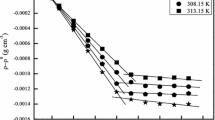
The effect of aliphatic and aromatic hydrocarbons on surfactant micellar growth has been investigated by viscosity measurements at 40°C. Aqueous and aqueous KBr (0.1 M) solutions of 0.1 M cetylpyridinium bromide (CPB) showed that the viscosity behavior changed substantially in the presence of KBr. This is attributed to favorable conditions produced by KBr that assist micellar growth by addition of hydrocarbons. Reasons for the effectiveness of the solubilized hydrocarbons are suggested and supported by theoretical arguments. The causes of viscosity decrease at higher aromatic hydrocarbon concentrations are also explained. Micellar growth with soluble aromatic/aliphatic hydrocarbons could also be initiated if a moderate salt concentration is present in CPB micellar solutions. The chainlength, solubilization site, and molar volume of the soluble hydrocarbons all affect the bulk viscosity of the solution. Such surfactant and hydrocarbon combinations may find use in micellar-enhanced ultrafiltration of benzene and its derivatives, but it should be kept in mind that micellar shape may change and be more curved at higher benzene derivative concentrations.
Similar content being viewed by others
References
Rao, U.R.K., C. Manohar, B.S. Valaulikar, and R.M. Iyer, On the Origin of Viscoelasticity in Micellar Solutions of Cetyltrimethylammonium Bromide and Sodium Salicylate, Ibid.:3286–3292 (1987).
Stigter, D., On Density, Hydration, Shape, and Charge of Micelles of Sodium Dodecyl Sulfate and Dodecylammonium Chloride, J. Colloid Interface Sci. 23:379–388 (1967).
Lindemuth, P.M., and G.L. Bertrand, Calorimetric Observations of the Transition of Spherical to Rodlike Micelles with Solubilized Organic Additives, J. Phys. Chem. 97:7769–7773 (1993).
Kumar, S., V.K. Aswal, H.N. Singh, P.S. Goyal, and Kabir-ud-Din, Growth of Sodium Dodecyl Sulfate Micelles in the Presence of n-Octylamine, Langmuir 10:4069–4072 (1994).
Israelachvili, J.N., D.J. Mitchell, and B.W. Ninham, Theory of Self-Assembly of Hydrocarbon Amphiphiles into Micelles and Bilayers, J. Chem. Soc., Faraday Trans. 2 72:1525–1568 (1976).
Bohmer, M.R., L.K. Koopal, and J. Lyklema, Micellization of Ionic Surfactants. Calculations Based on a Self-Consistent Field Lattice Model, J. Phys. Chem. 95:9569–9578 (1991).
Mitchell, D.J., and B.W. Ninham, Micelles, Vesicles and Microemulsions, J. Chem. Soc., Faraday Trans. 2 77:601–629 (1980).
Tanford, C., Micelle Shape and Size, J. Phys. Chem. 76:3020–3024 (1972).
Fang, J., Article II: Ph.D. Dissertation, University of Missouri-Rolla, 1987.
Ozeki, S., and S. Ikeda, The Sphere-Rod Transition of Micelles and the Two Step Micellization of Dodecyltrimethylammonium Bromide in Aqueous NaBr Solutions, J. Colloid Interface Sci. 87:424–435 (1982).
Khatory, A., F. Kern, F. Lequeux, J. Appell, G. Porte, N. Morie, A. Ott, and W. Urbach, Entangled Versus Multiconnected Network of Wormlike Micelles, Langmuir 9:933–939 (1993).
Kumar, S., Kirti, K. Kumari, and Kabir-ud-Din, Role of Alkanols in Micellar Growth: A Viscometric Study, J. Am. Oil Chem. Soc. 72:817–821 (1995).
Kabir-ud-Din, S. Kumar, Kirti, and P.S. Goyal, Micellar Growth in Presence of Alcohols and Amines: A Viscometric Study, Langmuir 12:1490–1494 (1996).
David, S.L., S. Kumar, and Kabir-ud-Din, Viscosities of Cetylpyridinium Bromide Solutions (Aqueous and Aqueous KBr) in the Presence of Alcohols and Amines, J. Chem. Eng. Data 42:198–201 (1997).
Winsor, P.A., Hydrotropy, Solubilization and Related Emulsification Processes, Trans. Faraday Soc. 2. 44:376–398 (1948).
Eriksson J.C., and G. Gillberg, NMR Studies of the Solubilization of Aromatic Compounds in Cetyltrimethylammonium Bromide Solution II, Acta Chem. Scand. 20:2019–2027 (1966).
Venable, R.L., K.L. Elders, and J. Fang, Microemulsions with High Water Solubilizing Capacity at High Hydrocarbon Levels and Very Low Surfactant Concentrations, J. Colloid Interface Sci. 109:330–335 (1986).
Abe, M., D. Schechter, R.S. Schechter, W.H. Wade, U. Weersdooriya, and S. Xiv, Microemulsion Formation with Branched Tail Polyoxyethlene Sulfonate Surfactants, Ibid.:342–356 (1986).
Almgren, M., and S. Swarup, Size of Sodium Dodecyl Sulfate Micelles in the Presence of Additives. 2. Aromatic and Saturated Hydrocarbons, J. Phys. Chem. 86:4212–4216 (1982).
Ozeki, S., and S. Ikeda, The Viscosity Behavior of Aqueous NaCl Solutions of Dodecyl Dimethyl Ammonium Chloride and the Flexibility of its Rod-like Micelle, J. Colloid Interface Sci. 77:219–231 (1980).
Ogino, K., and N. Takeshita, Electrophoresis and Diffusion Studies of the Solubilization of Polar and Nonpolar Oily Compounds with Sodium Dodecyl Sulfate. II, Bull. Chem. Soc. Jpn. 53:611–615 (1980).
Rosen, M.J., Surfactants and Interfacial Phenomena, Wiley, New York, 1989.
Lindblom, G., B. Lindman, and L. Mandell, Effect of Micellar Shape and Solubilization on Counterion Binding Studied by 81Br NMR, J. Colloid Interface Sci. 42:400–409 (1973).
Hoffmann, H., Fascinating Phenomena in Surfactant Chemistry, Adv. Colloid Interface Sci. 32:123–150 (1990).
Mukerjee, P., Formation and Some Properties of Micelles, Ber Bunsenges. Phys. Chem. 82:931–936 (1978).
Gadelle, F., W.J. Koros, and R.S. Schechter, Solubilization Isotherms of Aromatic Solutes in Surfactant Aggregates, J. Colloid Interface Sci. 170:57–64 (1995).
Author information
Authors and Affiliations
About this article
Cite this article
Kumar, S., David, S.L. & Kabir-ud-Din Effects of various hydrocarbons on micellar growth. J Amer Oil Chem Soc 74, 797–801 (1997). https://doi.org/10.1007/s11746-997-0221-y
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11746-997-0221-y