Abstract
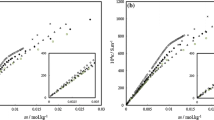
We have measured the viscosity of solutions of 0.3 M sodium dodecyl sulfate (SDS) + 0.3 M NaBr +n-alkanols as a function of [alkanol] and temperature. When propanol was added, the viscosity of micellar solutions remained almost constant and then decreased, whereas it continuously increased with hexanol. However, with butanol or pentanol, depending upon the added concentration, increases followed by decreases in viscosity were observed. This behavior has been discussed in light of solubility of alkanols in various soluble phases of the micellar system with a resultant change in the Mitchell-Ninham parameter of the “effective surfactant” (i.e., SDS +n-alkanol). An increase in temperature caused a decrease in viscosity, which is related to micellar breakdown. Activation parameters (ΔG* and ΔH*) were computed from the temperature dependence data. ΔH* Covered almost the total contribution to ΔG*.
Similar content being viewed by others
References
Mittal, K.L.,Micellization, Solubilization and Microemulsions, Vols. 1 and 2, Plenum Press, New York, 1977.
Shah, O.H. (ed.),Surface Phenomena in Enhanced Oil Recovery, Plenum Press, New York, 1981.
Rosen, M.J. (ed.),Surfactants in Emerging Technologies, Marcel Dekker, New York, 1987.
Armstrong, D.W., and W.L. Hinze (eds.),Use of Ordered Media in Chemical Separations, ACS Symposium Series 342, American Chemical Society, Washington D.C., 1987.
Wang, J.,Colloids and Surfaces 70:15 (1993).
Kumar, S., Kirti, and Kabir-un-Din,J. Am. Oil Chem. Soc. 71:763 (1994).
Lucassen-Reynders, E.H.,Anionic Surfactant: Physical Chemistry of Surfactant Action, Vol. 11, Surfactant Science Series, Marcel Dekker, New York, 1981, Chapter 2.
Mitchell, D.J., and B.W. Ninham,J. Chem. Soc., Faraday Trans. 277:601 (1981).
Hertel, G., and H. Hoffman,Liq. Cryst. 5:1883 (1989).
Guerin, G., and A.M. Bellocq,J. Phys. Chem. 92:2550 (1988).
Almgren, M., and J.E. Lofroth,J. Colloid Interface Sci. 81:486 (1981).
Lianos, P., J. Lang, C. Strazielle and R. Zana,J. Phys. Chem. 86:1019 (1982).
Almgren, M., and S. Swarup,J. Colloid Interface Sci. 91:256 (1983).
Croonen, Y., E. Gelade, M. van der Zegel, M. van der Auweraer, H. Vandendriessche, F.C. De Schryver and M. Almgren,J. Phys. Chem. 87:1426 (1983).
Hoeiland, H., O. Kvammen, S. Backlund and K. Rundt, inSurfactants in Solution, edited by K.L. Mittal, and B. Lindman, Plenum Press, New York, 1982.
Nguyen, D., and G.L. Bertrand,J. Phys. Chem. 96:1994 (1992).
Lindemuth, P.M., and G.L. Bertrand, Ibid.:7769 (1993).
Stephany, S.M., T.M. Kole and M.R. Fisch, Ibid.:11126 (1994).
Gamboa, C., and L. Sepulveda,J. Colloid Interface Sci. 113:566 (1986).
Ozeki, S., and S. Ikeda, Ibid.:219 (1980).
Hayase, K., and S. Hayano,Bull. Chem. Soc. Japan 50:83 (1977).
Bayer, O., H. Hoffman and W. Ulbricht, inSurfactants in Solution, Vol. 4, edited by K.L. Mittal, and P. Bothorel, Plenum Press, New York, 1986.
Backlund, S., J. Bakken, A.M. Blokhus, H. Hoeiland and I. Vikholm,Acta Chem. Scand. A40:241 (1986).
Tominaga, T., T.B. Stem and D.F. Evans,Bull. Chem. Soc. Japan 53:795 (1980).
Hirsch, E., S. Candau and R. Zana,J. Colloid Interface Sci. 97:318 (1984).
Prasad, Ch.D., and H.N. Singh,Colloids and Surfaces 50:37 (1990).
Mishra, B.K., S.D. Samant, P. Paradhan, S.B. Mishra and C. Manohar,Langmuir 9:894 (1993).
Glasstone, S., K.J. Laidler and H. Eyring,The Theory of Rate Processes, McGraw-Hill, New York, 1941.
Lindman, B., and H. Wennerstrom,Micelles: Topics in Current Chemistry, Springer-Verlag, Berlin/Heidelberg/New York, 1980.
Rehage, H., and H. Hoffman,J. Phys. Chem. 92:4712 (1988).
Author information
Authors and Affiliations
About this article
Cite this article
Kumar, S., Kirti, Kumari, K. et al. Role of alkanols in micellar growth: A viscometric study. J Am Oil Chem Soc 72, 817–821 (1995). https://doi.org/10.1007/BF02541031
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02541031