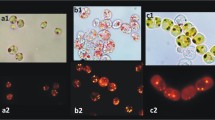
Abstract
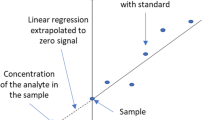
Nile red staining has been used as a lipid quantification technique in many microalgae growth and oil accumulation studies. However, its application in lysed microalgae cells is limited. Therefore, this study focused on lysed microalgae cells and utilized the Nile red staining technique to evaluate oil content and extraction. This study aims to provide a rapid and high-throughput alternative method particularly in the microalgae extraction screening process. Potential interferences such as chlorophyll, β-carotene, soluble protein, and phospholipids were evaluated. The hydrophobic Nile red dye was found to quench in water, therefore the fluorometric measurement has to be completed immediately or within 5 min of dye addition. The fluorescence intensity was also found to be Nile red concentration dependent. The optimum Nile red concentration of 656 ppb was used throughout the study. In microalgae samples containing chlorophyll and carotenoids (such as Nannochloropsis sp.), Nile red fluorescence intensity was significantly reduced in comparison to non-chlorophyll microalgae (Schizochytrium limacinum). Soluble proteins from defatted microalgae did not fluoresce significantly relative to lipids, therefore did not interfere with the method to a high degree. Comparing the optimized Nile red staining method with the gravimetric lipid quantification method, a good linear correlation was found in all three materials tested (soybean oil, Nannochloropsis sp., and Schizochytrium limacinum).











Similar content being viewed by others
References
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sust Energ Rev 14:217–232
Brennan L, Owende P (2010) Biofuels from microalgae––a review of technologies for production, processing and extractions of biofuels and co-products. Renew Sust Energ Rev 14:557–577
Bligh EG, Dyer WJ (1959) A rapid method for total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Cooksey KE, Guckert JB, Williams SA, Callis PR (1987) Fluorometric determination of the neutral lipid content of microalgal cells using Nile red. J Microbiol Methods 6:333–345
Halim R, Danquah MK, Webley PA (2012) Extraction of oil from microalgae for biodiesel production: a review. Biotechnol Adv 30:709–732
Lee JY, Yoo C, Jun SY, Ahn CY, Oh HM (2010) Comparison of several methods for effective lipid extraction from microalgae. Bioresour Technol 101:S75–S77
Mercer P, Armenta RE (2011) Developments in oil extraction from microalgae. Eur J Lipid Sci Technol 113:539–547
Greenspan P, Fowler SD (1985) Spectrofluorometric studies of the lipid probe, Nile red. J Lipid Res 26:781–789
Chen W, Zhang C, Song L, Sommerfield M, Hu Q (2009) A high throughput Nile red method for quantitative measurement of neutral lipids in microalgae. J Microbiol Methods 77:41–47
Huang GH, Chen G, Chen F (2009) Rapid screening method for lipid production in alga based on Nile red fluorescence. Biomass Bioenerg 33:1386–1392
Chen W, Sommerfield M, Hu Q (2011) Microwave assisted Nile red method for in vivo quantification of neutral lipids in microalgae. Bioresour Technol 102:135–141
Doan TTY, Obbard JP (2011) Improved Nile red staining of Nannochloropsis sp. J Appl Phycol 23:895–901
Pick U, Rachutin-Zalogin T (2012) Kinetic anomalies in the interactions of Nile red with microalgae. J Microbiol Methods 88:189–196
Gerde J, Montalbo-Lomboy M, Yao L, Grewell D, Wang T (2012) Evaluation of microalgae cell disruption by ultrasonic treatment. Bioresour Technol 125:175–181
Wang G, Wang T (2012) Characterization of lipid components in two microalgae for biofuel application. J Am Oil Chem Soc 89:135–143
Johnson MB, Wen Z (2009) Production of biodiesel fuel from the microalga Schizochytrium limacinum by direct transesterification of algal biomass. Energ Fuel 23:5179–5183
Ethier S, Woisard K, Vaughan D, Wen Z (2011) Continuous culture of the microalgae Schizochytrium limacinum on biodiesel-derived crude glycerol for producing docosahexaenoic acid. Bioresour Technol 102:88–93
Armenta RE, Valentine MC (2013) Single-cell oils as a source of omega-3 fatty acids: an overview of recent advances. J Am Oil Chem 90:167–182
Folch J, Lees M, Stanley S (1956) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226(1):497–509
Bradford M (1976) A rapid and sensitive method for the quantification of microgram of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Lakowicz JR (1999) Principles of fluorescence spectroscopy, 2nd edn. Springer Science, New York
Schoefs B (2002) Chlorophyll and carotenoid analysis in food products. Properties of the pigments and method of analysis. Trends Food Sci Technol 13:361–371
Cerovic ZG, Ounis A, Cartelat A, Latouche G, Goulas Y, Meyer S, Moya I (2002) The use of chlorophyll fluorescence excitation spectra for the nondestructive in site assessment of UV-absorbing compounds in leave. Plant Cell Environ 25:1663–1676
Gillbro T, Cogdell R (1989) Carotenoid fluorescence. Chem Phys Lett 158(3,4):312–316
Archanaa S, Moise S, Suraishkumar GK (2012) Chlorophyll interference in microalgal lipid quantification through the Bligh and Dyer method. Biomass Bioenerg 46:805–808
Acknowledgments
The authors would like to thank Dr. Zhiyou Wen for providing assistance in the cultivation of Schizochytrium limacinum cells. Sincere gratitude is also extended to Dr. Linxing Yao and Dr. Peiyou Qin for their support and technical recommendations.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Montalbo-Lomboy, M., Kantekin, M.N. & Wang, T. Lipid Estimation of Surfactant-Extracted Microalgae Oil Using Nile Red. J Am Oil Chem Soc 91, 665–680 (2014). https://doi.org/10.1007/s11746-013-2395-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-013-2395-9