Abstract
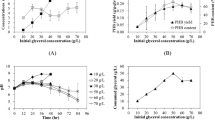
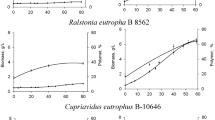
One refined and two crude glycerol (from biodiesel production) samples were utilized to produce poly(3-hydroxybutyrate) (PHB) by Pseudomonas oleovorans NRRL B-14682. A batch culture fermentation protocol including 1% glycerol and an aeration rate of 3 standard liters per minute proved best for PHB synthesis (av. yield = 1.0 ± 0.2 g/L at 48 h) and efficient glycerol utilization. PHB molecular weights decreased as MeOH concentration increased. Refined glycerol resulted in PHB polymers with number average molecular weights (M n) of 314,000 g/mol which decreased by 17 and 90% as MeOH media concentrations increased to <0.005 and 0.85%, respectively. Proton (1H) NMR demonstrated the presence of glycerol- and methoxy-based end-capping, which was confirmed by 1H diffusion experiments (DOSY analyses). NMR diffusion analyses of the PHB polymers established their diffusivities, and confirmed that their relative molecular sizes were dependent on the impurities in the glycerol. In addition, DOSY analyses indicated that each end-capped PHB polymer and the glycerol or methoxy groups bound to it had the same diffusion constants, demonstrating that they migrated together as covalent complexes. Non-covalent complexation was eliminated by physically mixing free glycerol with PHB synthesized from oleic acid; their respective diffusivities were notably faster.








Similar content being viewed by others
References
Lin Y, Tanaka S (2006) Ethanol fermentation from biomass resources: current state and prospects. Appl Microbiol Biotechnol 69:627–642
Taherzadeh M, Karimi K (2008) Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. Int J Mol Sci 9:1621–1651
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Lardon L, Hélias A, Sialve B, Steyer JP, Bernard O (2009) Life-cycle assessment of biodiesel production from microalgae. Environ Sci Technol 43:6475–6481
Adamczak M, Bornscheuer UT, Bednarski W (2009) The application of biotechnological methods for the synthesis of biodiesel. Eur J Lipid Sci Technol 111:808–813
Papanikolaou S, Ruiz-Sanchez P, Pariset B, Blanchard F, Fick M (2000) High production of 1, 3 propanediol from industrial glycerol by a newly isolated Clostridium butyricum strain. J Biotechnol 77:191–208
Mu Y, Teng H, Zhang DJ, Wang W, Xiu ZL (2006) Microbial production of 1,3 propanediol by Klebsiella pneumoniae using crude glycerol from biodiesel preparations. Biotechnol Lett 28:1755–1759
Oh BR, Seo JW, Choi MH, Kim CH (2008) Optimization of culture conditions for 1,3-propanediol production from crude glycerol by Klebsiella pneumoniae using response surface methodology. Biotechnol Bioprocess Eng 13:666–670
Ito T, Nakashimada Y, Senba K, Matsui T, Nishio N (2005) Hydrogen and ethanol production from glycerol-containing wastes discharged after biodiesel manufacturing process. J Biosci Bioeng 100:260–265
Scholten E, Renz T, Thomas J (2009) Continuous cultivation approach for fermentative succinic acid production from crude glycerol by Basfia succiniciproducens DD1. Biotechnol Lett 31:1947–1951
Ashby RD, Nuñez A, Solaiman DKY, Foglia TA (2005) Sophorolipid biosynthesis from a biodiesel co-product stream. J Am Oil Chem Soc 82:625–630
Rywińska A, Rymowicz W (2009) Citric acid production from raw glycerol by Yarrowia lipolytica wratislavia 1.31. In: Aggelis G (ed) Microbial conversions of raw glycerol. Nova Science Publishers Inc, New York, pp 19–30
Papanikolaou S, Muniglia L, Chevalot I, Aggelis A, Marc I (2002) Yarrowia lipolytica as a potential producer of citric acid from raw glycerol. J Appl Microbiol 92:737–744
Papanikolaou S, Aggelis G (2009) Biotechnological valorization of biodiesel derived glycerol waste through production of single cell oil and citric acid by Yarrowia lipolytica. Lipid Technol 21:83–87
Huijberts GNM, Eggink G, de Waard P, Huisman GW, Witholt B (1992) Pseudomonas putida KT2442 cultivated on glucose accumulates poly(3-hydroxyalkanoates) consisting of saturated and unsaturated monomers. Appl Environ Microbiol 58:536–544
Eggink G, van der Wal H, Huijberts GNM, de Waard P (1993) Oleic acid as a substrate for poly-3-hydroxybutyrate formation in Alcaligenes eutrophus and Pseudomonas putida. Ind Crops Prod 1:157–163
de Smet MJ, Eggink G, Witholt B, Kingma J, Wynberg H (1983) Characterization of intracellular inclusions former by Pseudomonas oleovorans during growth on octane. J Bacteriol 154:870–878
Ashby RD, Foglia TA (1998) Poly(hydroxyalkanoate) biosynthesis from triglyceride substrates. Appl Microbiol Biotechnol 49:431–437
Solaiman DKY, Ashby RD, Foglia TA (2002) Physiological characterization and genetic engineering of Pseudomonas corrugata for medium-chain-length polyhydroxyalkanoates synthesis from triacylglycerols. Curr Microbiol 44:189–195
Solaiman DKY, Ashby RD, Hotchkiss AT, Foglia TA (2006) Biosynthesis of medium-chain-length poly(hydroxyalkanoates) from soy molasses. Biotechnol Lett 28:157–162
Ashby RD, Solaiman DKY, Foglia TA (2004) Bacterial poly(hydroxyalkanoate) polymer production from the biodiesel co-product stream. J Polym Environ 12:105–112
Cavalheiro JMBT, de Almeida MCMD, Grandfils C, da Fonseca MMR (2009) Poly(3-hydroxybutyrate) production by Cupriavidus necator using waste glycerol. Proc Biochem 44:509–515
Shrivastav A, Mishra SK, Shethia B, Pancha I, Jain D, Mishra S (2010) Isolation of promising bacterial strains from soil and marine environment for polyhydroxyalkanoates (phas) production utilizing Jatropha biodiesel byproduct. Int J Biol Macromol 47:283–287
Zhu C, Nomura CT, Perrota J, Stipanovic AJ, Nakas JP (2010) Production and characterization of poly-3-hydroxybutyrate from biodiesel-glycerol by Burkholderia cepacia ATCC17759. Biotechnol Prog 26:424–430
Mothes G, Schnorpfeil C, Ackermann JU (2007) Production of PHB from crude glycerol. Eng Life Sci 7:475–479
Kawata Y, Aiba S (2010) Poly(3-hydroxybutyrate) production by isolated Halomonas sp. KM-1 using waste glycerol. Biosci Biotechnol Biochem 74:175–177
Koutinas AA, Xu YX, Wang R, Webb C (2007) Polyhydroxybutyrate production from a novel feedstock derived from a wheat-based biorefinery. Enzyme Microb Technol 40:1035–1044
Xu Y, Wang RH, Koutinas AA, Webb C (2010) Microbial biodegradable plastic production from a wheat-based biorefining strategy. Proc Biochem 45:153–163
Foglia TA, Jones KC (1997) Quantitation of neutral lipid mixtures using high performance liquid chromatography with light scattering detection. J Liq Chrom Relat Tech 20:1829–1838
Ashby RD, Solaiman DKY, Foglia TA, Liu CK (2001) Glucose/lipid mixed substrates as a means of controlling the properties of medium chain length poly(hydroxyalkanoates). Biomacromolecules 2:211–216
Brandl H, Gross RA, Lenz RW, Fuller RC (1988) Pseudomonas oleovorans as a source of poly(β-hydroxyalkanoates) for potential applications as biodegradable polyesters. Appl Environ Microbiol 54:1977–1982
Ashby RD, Foglia TA, Solaiman DKY, Liu CK, Nuñez A, Eggink G (2000) Viscoelastic properties of linseed oil-based medium chain length poly(hydroxyalkanoate) films: effects of epoxidation and curing. Int J Biol Macromol 27:355–361
Lee EY, Choi CY (1995) Gas chromatography–mass spectrometric analysis and its application to a screening procedure for novel bacterial polyhydroxyalkanoic acids containing long chain saturated and unsaturated monomers. J Ferm Bioeng 80:408–414
Pelta MD, Morris GA, Stchedroff MJ, Hammond S (2002) A one-shot sequence for high-resolution diffusion-ordered spectroscopy. Magn Reson Chem 40:S147–S152
Macchino A, Ciancaleoni G, Zuccaccia C, Zuccaccia D (2008) Determining accurate molecular sizes in solution through NMR diffusion spectroscopy. Chem Soc Rev 37:479–489
Price WS (1998) Pulsed-field gradient nuclear magnetic resonance as a tool for studying translational diffusion: part II. Experimental aspects. Concepts Magn Reson 10:197–237
Nilsson M (2009) The DOSY toolbox: a new tool for processing PFG NMR diffusion data. J Magn Reson 200:296–302
Alvarez HM, Kalscheuer R, Steinbuchel A (2000) Accumulation and mobilization of storage lipids by Rhodococcus opacus PD630 and Rhodococcus ruber NCIMB 40126. Appl Microbiol Biotechnol 54:218–223
Fakas S, Galiotou-Panayotou M, Papanikolaou S, Komaitis M, Aggelis G (2007) Compositional shifts in lipid fractions during lipid turnover in Cunninghamella echinulata. Enzyme Microb Technol 40:1321–1327
Ashby RD, Solaiman DKY, Foglia TA (2005) Synthesis of short-/medium-chain-length poly(hydroxyalkanoate) blends by mixed culture fermentation of glycerol. Biomacromolecules 6:2106–2112
Ashby RD, Solaiman DKY, Foglia TA (2002) Poly(ethylene glycol)-mediated molar mass control of short-chain and medium-chain-length poly(hydroxyalkanoates) from Pseudomonas oleovorans. Appl Microbiol Biotechnol 60:154–159
Ashby RD, Shi F, Gross RA (1997) Use of poly(ethylene glycol) to control the end group structure and molecular weight of poly(3-hydroxybutyrate) formed by Alcaligenes latus DSM 1122. Tetrahedron 53:15209–15223
Acknowledgments
The authors thank Bun Hong Lai, Jennifer Thomas, Aisha Abdul-Wakeel and Kirby Jones for their technical assistance throughout the study and Dr Chris Nomura of the Department of Chemistry, SUNY College of Environmental Science and Forestry, Syracuse, NY for performing the GPC analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture.
About this article
Cite this article
Ashby, R.D., Solaiman, D.K.Y. & Strahan, G.D. Efficient Utilization of Crude Glycerol as Fermentation Substrate in the Synthesis of Poly(3-hydroxybutyrate) Biopolymers. J Am Oil Chem Soc 88, 949–959 (2011). https://doi.org/10.1007/s11746-011-1755-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-011-1755-6