Abstract
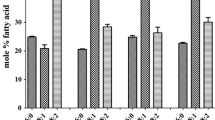
Cottonseed typically contains about 15% oleic acid. Here we report the development of transgenic cotton plants with higher seed oleic acid levels. Plants were generated by Agrobacterium-mediated transformation. A binary vector was designed to suppress expression of the endogenous cottonseed †-12 desaturase (fad2) by subcloning a mutant allele of a rapeseed fad2 gene downstream from a heterologous, seedspecific promoter (phaseolin). Fatty acid profiles of total seed lipids from 43 independent transgenic lines were analyzed by gas chromatography. Increased seed oleic acid content ranged from 21 to 30% (by weight) of total fatty acid content in 22 of the primary transformants. The increase in oleic acid content was at the expense of linoleic acid, consistent with reduced activity of cottonseed FAD2. Progeny of some lines yielded oleic acid content as high as 47% (three times that of standard cottonseed oil). Molecular analyses of nuclear DNA from transgenics confirmed the integration of the canola transgene into the cotton genome. Collectively, our results extend the metabolic engineering of vegetable oils to cottonseed and should provide the basis for the development of a family of novel cottonseed oils.
Similar content being viewed by others
References
Cottonseed: World's No. 3 Oilseed Yields Diverse Products, inform 11:820–839 (2000).
Jones, L.A., and C.C. King, Cottonseed Oil, in Bailey's Industrial Oil and Fat Products, Vol. 2 Edible Oil and Fat Products: Oils and Oil Seeds, 5th edn. edited by Y. Hui, John Wiley & Sons, New York, 1996, pp. 159–240.
Kinney, A.L., and S. Knowlton, Designer Oils:: The High-Oleic Soybean, in Genetic Modification in the Food Industry, edited by S. Roller and S. Harlander, Blackie Academic and Professional, New York, 1998, pp. 193–213.
Kinney, A.J., Genetic Engineering of the Storage Lipids of Plants, Curr. Opinion Biotechnol. 5:144–151 (1994).
Browse, J., J. Spychalla, J. Okuley, and J. Lightner, Altering the Fatty Acid Composition of Vegetable Oils, in Plant Lipid Biosynthesis: Fundamentals and Agricultural Applications, edited by J.L. Harwood, Society for Experimental Biology Seminar Series 67, Cambridge University Press, Cambridge, 1998, pp. 131–153.
Downey, R.K., and G. Röbbelen, Brassica Species, in Oil Crops of the World: Their Breeding and Utilization, edited by G. Röbbelen, R.K. Downey, and A. Ashri, McGraw Hill, New York, 1989, pp. 339–362.
Green, A.G., and D.R. Marshall, Isolation of Induced Mutants in Linseed (Linum usitatissimum) Having Reduced Linolenic Acid Content, Euphytica 33:321–328 (1984).
Kinney, A.J., Genetic Engineering of Oilseeds for Desired Traits, in Genetic Engineering, edited by J.K. Setlow, Plenum Press, New York 1997, Vol. 19, pp. 149–166.
Del Vecchio, A.J., High-Laurate Canola, inform 7:230–243 (1996).
Kinney, A.J., Production of Specialised Oils for Industry, in Plant Lipid Biosynthesis: Fundamentals and Agricultural Applications, edited by J.L. Harwood, Society for Experimental Biology Seminar Series 67, Cambridge University Press, Cambridge, 1998, pp. 273–285.
Mazur, B., E. Krebbers, and S. Tingey, Gene Discovery and Product Development for Grain Quality Traits, Science 285: 372–375 (1999).
Ohlrogge, J.B., Design of New Plant Products: Engineering of Fatty Acid Metabolism, Plant Physiol. 104:821–826 (1994).
Voelker, T., Plant Acyl-ACP Thioesterases: Chain-Length Determining Enzymes in Plant Fatty Acid Biosynthesis, in Genetic Engineering, edited by J.K. Setlow, Plenum Press, New York, 1996, Vol. 18, pp. 111–133.
Somerville, C., J. Browse, J. Jaworski, and J.B. Ohlrogge, Lipids, in Biochemistry and Molecular Biology of Plants, edited by B. Buchanan, W. Gruissem, and R. Jones, American Society of Plant Physiologists, Rockville, MD, 2000, pp. 456–527.
Harwood, J.L., Recent Advances in the Biosynthesis of Plant Fatty Acids, Biochim. Biophys. Acta 1301:7–56 (1996).
Mekhedov, S., O. Martínez de Ilárduya, and J.B. Ohlrogge, Toward a Functional Catalog of the Plant Genome. A Survey of Genes for Lipid Biosynthesis, Plant Physiol. 122:389–402 (2000).
Voelker, T.A., A.C. Worrell, L. Anderson, J. Bleibaum, C. Fan, D.J. Hawkins, S.E. Radke, and H.M. Davies, Fatty Acid Biosynthesis Redirected to Medium Chains in Transgenic Oilseed Plants, Science 257:72–74 (1992).
Shanklin, J., and E.B. Cahoon, Desaturation and Related Modifications of Fatty Acids, Annu. Rev. Plant Physiol. Plant Mol. Biol. 49:611–642 (1998).
Okuley, J., J. Lightner, K. Feldmann, N. Yadav, E. Lark, and J. Browse, Arabidopsis FAD2 Gene Encodes the Enzyme That Is Essential for Polyunsaturated Lipid Synthesis, Plant Cell 6:147–158 (1994).
Burow, M.D., P. Sen, C.A. Chlan, and N. Murai, Developmental Control of the beta-Phaseolin Gene Requires Positive, Negative, and Temporal Seed-Specific Transcriptional Regulatory Elements and a Negative Element for Stem and Root Expression, Plant J. 2:537–548 (1992).
Thomas, J.C., D.G. Adams, V.D. Keppenne, C.C. Wasmann, J.K. Brown, M.R. Kanost, and H.J. Bohnert, Protease Inhibitors of Madhuca sexta Expressed in Transgenic Cotton, Plant Cell Rep. 14:758–762 (1995).
Trolinder, N.L., and J.R. Goodin, Somatic Embryogenesis in Cotton (Gossypium). II. Requirements for Embryo Development and Plant Regeneration, Plant Cell Tissue Org. Cult. 12:43–53 (1988).
Umbeck, P., G. Johnson, K. Baron, and W. Swain, Genetically Transformed Cotton (Gossypium hirsutum L.), Plants Biotechnol. 5:263–266 (1987).
Firoozabady, E., D.L. DeBoer, D.J. Merlo, E.L. Halk, L.N. Amerson, K.E. Rashka, and E.E. Murray, Transformation of Cotton (Gossypium hirsutum L.) by Agrobacterium tumefaciens and Regeneration of Transgenic Plants, Plant Mol. Biol. 10:105–116 (1987).
Hemphill, J.K., C.G.A. Maier, and K.D. Chapman, Rapid in vitro Regeneration of Cotton (Gossypium hirsutum L.), Plant Cell Rep. 17:273–278 (1998).
Christie, W.W., Lipid Analysis, 2nd edn, Pergamon Press, New York, 1982, pp. 52–54.
Chapman, K.D., and R.N. Trelease, Acquisition of Membrane Lipids by Differentiating Glyoxysomes: Role of Lipid Bodies, J. Cell Biol. 115:995–1007 (1991).
Paterson, A.H., C.L. Brubaker, and J.F. Wendel, A Rapid Method for Extraction of Cotton (Gossypium spp.) Genomic DNA Suitable for RFLP or PCR Analysis, Plant Mol. Biol. Reporter 11:122–127 (1993).
Sambrook, J., E.F. Fritsch, and T. Maniatis, Molecular Cloning: A Laboratory Manual 2nd edn., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 1989.
Feinberg, A.P., and B. Vogelstein, A Technique for Radiolabeling DNA Restriction Endonuclease Fragments to High Specific Activity, Anal. Biochem. 132:6–13 (1983).
Reed, K.C., and D.A. Mann, Rapid Transfer of DNA from Agarose Gels to Nylon Membranes, Nucl. Acids Res. 13:7207–7221 (1985).
Trelease, R.N., J.A. Miernyk, J.S. Choinski, Jr., and S.J. Bortman, Synthesis and Compartmentation of Enzymes During Cottonseed Maturation, in Cotton Physiology, edited by J.M. Stewart, and J.R. Mauney, Cotton Foundation, Memphis, TN, 1986.
Croteau, R., T.M. Kutchan, and N.G. Lewis, Natural Products (secondary metabolites), in Biochemistry and Molecular Biology of Plants, edited by B. Buchannan, W. Gruissem, and R. Jones, American Society of Plant Physiologists, Rockville, MD, 2000, pp. 1250–1318.
Wood, R., Comparison of the Cyclopropene Fatty Acid Content of Cottoneed Varieties, Glanded and Glandless Seeds, and Various Seed Structures, Biochem. Arch. 2:73–80 (1986).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Chapman, K.D., Austin-Brown, S., Sparace, S.A. et al. Transgenic cotton plants with increased seed oleic acid content. J Amer Oil Chem Soc 78, 941–947 (2001). https://doi.org/10.1007/s11746-001-0368-y
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11746-001-0368-y