Abstract
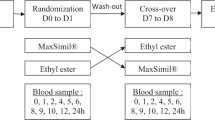
The effect of dietary docosahexaenoic acid (22∶6n−3, DHA) on the metabolism of oleic, linoleic, and linolenic acids was investigated in male subjects (n=6) confined to a metabolic unit and fed diets containing 6.5 or <0.1 g/d of DHA for 90 d. At the end of the diet period, the subjects were fed a mixture of deuterated triglycerides containing 18∶1n−9[d6], 18∶2n−6[d2], and 18∶3n−3[d4]. Blood samples were drawn at 0, 2, 4, 6, 8, 12, 24, 48, and 72 h. Methyl esters of plasma total lipids, triglycerides, phospholipids, and cholesterol esters were analyzed by gas chromatography-mass spectrometry. Chylomicron triglyceride results show that the deuterated fatty acids were equally well absorbed and diet did not influence absorption. Compared to the low-DHA diet (LO-DHA), clearance of the labeled fatty acids from chylomicron triglycerides was modestly higher for subjects fed the high DHA diet (HI-DHA). DHA supplementation significantly reduced the concentrations of most n-6[d2] and n-3[d4] long-chain fatty acid (LCFA) metabolites in plasma lipids. Accumulation of 20∶5n−3[d4] and 22∶6n−3[d4] was depressed by 76 and 88%, respectively. Accumulations of 20∶3n−6[d2] and 20∶4n−6[d2] were both decreased by 72%. No effect of diet was observed on acyltransferase selectivity or on uptake and clearance of 18∶1n−9[d6], 18∶2n−6[d2], and 18∶3n−3[d4]. The results indicate that accumulation of n−3 LCFA metabolites synthesized from 18∶3n−3 in typical U.S. diets would be reduced from about 120 to 30 mg/d by supplementation with 6.5 g/d of DHA. Accumulation of n−6 LCFA metabolites synthesized from 18∶2n−6 in U.S. diets is estimated to be reduced from about 800 to 180 mg/d. This decrease is two to three times the amount of n−6 LCFA in a typical U.S. diet. These results support the hypothesis that health benefits associated with DHA supplementation are the combined result of reduced accretion of n−6 LCFA metabolites and an increase in n−3 LCFA levels in tissue lipids.
Similar content being viewed by others
Abbreviations
- C18:
-
18-carbon chain length
- DHA:
-
docosahexaenoic acid
- EPA:
-
eicosapentaenoic acid
- GC:
-
gas chromatography
- HI-DHA:
-
high docosahexaenoic acid diet
- LCFA:
-
long-chain fatty acid
- LO-DHA:
-
low docosahexaenoic acid diet
- MS:
-
mass spectroscopy
- TLC:
-
thin-layer chromatography
References
Nettleton, J.A. (1995) Omega-3 Fatty Acids and Health, Chapman and Hall, New York, pp. 187–354.
Gerster, H. (1998) Can Adults Adequately Convert α-Linolenic Acid (18∶3n−3) to Eicosapentaenoic Acid (20∶5n−3) and Docosahexaenoic Acid (22∶6n−3)? Internat. J. Vit. Nutr. Res. 68, 159–173.
Nelson, G.J., Schmidt, P.S., Bartolini, G.L., Kelly, D.S., and Kyle, D. (1997) The Effect of Dietary Docosahexaenoic Acid on Platelet Funtion, Platelet Fatty Acid Composition, and Blood Coagulation in Humans, Lipids 32, 1129–1136.
Nelson, G.J., Schmidt, P.S., Bartolini, G.L., Kelly, D.S., and Kyle, D. (1997) The Effect of Dietary Docosahexaenoic Acid on Plasma Lipoproteins and Tissue Fatty Acid Composition in Humans, Lipids 32, 1137–1146.
Kelly, D.S., Taylor, P.C., Nelson, G.J., and Mackey, B.E. (1998) Dietary Docosahexaenoic Acid and Immunocompetence in Young Healthy Men, Lipids 33, 559–566.
Ferretti A., Nelson, G.J., Schmidt, P.S., Bartolini, G., Kelly, D.S., and Flanagan, V.P. (1998) Dietary Docosahexaenoic Acid Reduces the Thromboxane/Prostacyclin Synthesis Ratio in Humans, J. Nutr. Biochem. 6, 89–82.
Chen, Q., and Nilsson, A. (1993) Desaturation and Chain Elongation of n−3 and n−6 Polyunsaturated Fatty Acids in the Human CaCo-2 Cell Lines, Biochim. Biophys. Acta 1166, 193–201.
Emken, E.A., Adlof, R.O., Duval, S.M., and Nelson, G.J. (1998) Effect of Dietary Arachidonic Acid on Metabolism of Deuterated Linoleic Acid by Adult Male Subjects, Lipids 33, 471–480.
Emken, E.A., Adlof, R.O., Durval, S.M., and Nelson, G.J. (1997) Influence of Dietary Arachidonic Acid on Metabolism in vivo of 8c,11c,14c-Eicosatrienoic Acid in Humans, Lipids 32, 441–448.
Emken, E.A., Adlof, R.O., Duval, S.M., and Nelson, G.J. (1996) Influence of Dietary Arachidonic Acid on Human Metabolism of Deuterium-Labeled Dihomo-γ-linoleic Acid (20∶3n−6) and Arachidonic Acid (20∶4n−6), presented at the American Oil Chemists' Society meeting on “PUFA in Infant Nutrition: Consensus and Controversies,” held November 7–9, 1996, in Barcelona, Spain, p. 25, Meeting abstracts.
Consumer and Food Economic Institute (1992), The Composition of Foods—Raw, Processed, Prepared (Releases and Supplements), in USDA Agriculture Handbook 8, Nutrient Data Laboratory, U.S. Department of Agriculture, Washington, DC.
Adlof, R.O., and Emken, E.A. (1978) Synthesis of Methyl cis-9-Octadecenoate-14, 14,15,15,17,18-D6, J. Labelled Compd. Radiopharm. 15, 97–104.
Adlof, R.O., and Emken, E.A. (1993) Large-Scale Preparation of Linoleic Acid-d2 Enriched Triglycerides from Crepis alpina Seed Oil, J. Am. Oil. Chem. Soc. 70, 817–819.
Adlof, R.O., and Emken, E.A. (1987) Preparation of Methyl 8c,11c-Eicosadienoate-17,17,18,18-d4, Methyl 8c,11c,14c-Eicosatrienoate-17, 17,18,18-d4, J. Labelled Compd. Radiopharm. 24, 699–711.
Lindgren, F.T., Jensen, L.C., and Hatch, F.T. (1972) The Isolation and Quantitative Analysis of Serum Lipoproteins, in Blood Lipids and Lipoproteins (Nelson, G.J., ed.), pp. 186–188, Wiley-Interscience, New York.
Narayan, K.A. (1975) Electrophoresis Methods for the Separation of Serum Lipoproteins, in Analysis of Lipids and Lipoproteins (Perkins, E.G., ed.) pp. 225–249, American Oil Chemists' Society, Champaign.
Folch, J., Lees, M., and Sloane-Stanley, G.E. (1957) A Simple Method for the Isolation and Purification of Total Lipids from Animal Tissues, J. Biol. Chem. 226, 497–509.
French, J.A., and Anderson, D.W. (1973) Separation and Quantitative Recovery of Lipid Classes: A Convenient Thin-Layer Chromatographic Method, J. Chromatogr. 80, 133–136.
Christie, W.W. (1973) Lipid analysis, pp. 85–102, Pergamon Press Ltd., New York.
Rohwedder, W.K., Emken, E.A., and Wolf, D.J. (1985) Analysis of Deuterium-Labeled Blood Lipids by Chemical Ionization Mass Spectrometry, Lipids 20, 303–311.
Schenck, P.A., Rakoff, H., and Emken, E.A. (1996) Δ8 Desaturation in vivo of Deuterated Eicosatrienoic Acid by Mouse Liver, Lipids 31, 593–600.
Statistical Analysis System Institute (1987) SAS Guide for Personal Computers, 6th edn., SAS Institute Inc., Cary.
Emken, E.A., Adlof, R.O., Rohwedder W.K., and Gulley, R.M. (1994) Dietary Linoleic Acid Influences Desaturation and Acylation of Deuterium-Labeled Linoleic and Linolenic Acids in Young Adult Males, Biochim. Biophys. Acta 1213, 277–288.
Harris, W.S. (1996) n−3 Fatty Acids and Lipoproteins: Comparison of Results from Human and Animal Studies, Lipids 31, 243–252.
Agren, J.J., Hanninen, O., Julkunen, A., Fogelholm, L., Vidgren, H., Schwab, U., Pynnonen, O., and Uusitupa, M. (1996) Fish Diet, Fish Oil, and Docosahexaenoic Acid-Rich Oil Lower Fasting and Postprandial Plasma Lipid Levels, Eur. J. Clin. Nutr. 50, 765–771.
Patsch, J.R. (1994) Triglyceride-Rich Lipoproteins and Atherosclerosis, Atherosclerosis 110 (Suppl.), S23-S26.
Gotto, A.M. (1998) Triglyceride as a Risk Factor for Coronary Artery Disease, Am. J. Cardiol. 82, Q22-Q25.
Weintraub, M., Charach, G., and Grosskopf, I. (1997) Disturbances in Dietary Fat Metabolism and Their Role in the Development of Atherosclerosis, Biomed. Pharmacother. 51, 311–313.
National Research Council (1988) in Designing Foods: Animal Product Options in the Marketplace, pp. 45–62, National Academy Press, Washington, DC.
Nichamann, M.Z. (Chairman) (1989) Nutrition Monitoring in the United States: An Update Report on Nutrition Monitoring, DHHS Publication No. 89-1255, Life Science Research Office, Hyatsville, MD.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Emken, E.A., Adlof, R.O., Duval, S.M. et al. Effect of dietary docosahexaenoic acid on desaturation and uptake in vivo of isotope-labeled oleic, linoleic, and linolenic acids by male subjects. Lipids 34, 785–791 (1999). https://doi.org/10.1007/s11745-999-0424-2
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11745-999-0424-2