Abstract
Dietary intake of linoleic acid (LNA, 18:2n-6) has increased dramatically during the 20th century and is associated with greater prevalence of obesity. The endocannabinoid system is involved in regulation of energy balance and a sustained hyperactivity of the endocannabinoid system may contribute to obesity. Arachidonic acid (ARA, 20:4n-6) is the precursor for 2-AG and anandamide (AEA), and we sought to determine if low fat diets (LFD) could be made obesogenic by increasing the endocannabinoid precursor pool of ARA, causing excessive endocannabinoid signaling leading to weight gain and a metabolic profile associated with obesity. Mice (C57BL/6j, 6 weeks of age) were fed 1 en% LNA and 8 en% LNA in low fat (12.5 en%) and medium fat diets (MFD, 35 en%) for 16 weeks. We found that increasing dietary LNA from 1 to 8 en% in LFD and MFD significantly increased ARA in phospholipids (ARA–PL), elevated 2-AG and AEA in liver, elevated plasma leptin, and resulted in larger adipocytes and more macrophage infiltration in adipose tissue. In LFD, dietary LNA of 8 en% increased feed efficiency and caused greater weight gain than in an isocaloric reduction to 1 en% LNA. Increasing dietary LNA from 1 to 8 en% elevates liver endocannabinoid levels and increases the risk of developing obesity. Thus a high dietary content of LNA (8 en%) increases the adipogenic properties of a low fat diet.
Similar content being viewed by others
Introduction
The endocannabinoid signaling system includes the endogenous ligands 2-arachidonoylglycerol (2-AG) and N-arachidonoylethanolamine (anandamide or AEA) [1–4] and the cannabinoid receptors (CB1 and CB2) [5, 6]. Genetic and pharmacologic impairment of the CB1 receptor have demonstrated the role of the endocannabinoid system in energy homeostasis by inhibiting food intake and reducing body weight, both occurring independent of energy intake [7–9]. Activation of the cannabinoid receptor CB1 by endocannabinoids or exogenous agonists, centrally and peripherally, favors metabolic processes that stimulate appetite, increase food intake, activates fat storage pathways and down regulates catabolism resulting in adipose accretion [7, 10]. In the liver, CB1 activation increases de novo lipogenesis through stimulation of fatty acid synthase activity leading to fatty liver and obesity [10]. Under normal circumstances the endocannabinoid system is only activated on demand in response to acute stimulation, but has been found to be tonically overactivated in animal models of genetic and diet-induced obesity [10–15]. Down regulation of excessive endocannabinoid system activity is being actively pursued to reduce obesity [16]. Because pharmacological blockade of the CB1 receptor is effective in treating obesity and related metabolic derangements [17, 18], a dietary approach to diminish endocannabinoid hyperactivity may represent a safer alternative than pharmaceuticals [19, 20]. In this study, we are addressing an underlying cause of endocannabinoid overactivation that can potentially be translated into a safe alternative to prevent human obesity.
Current dietary guidelines recommend the replacement of saturated fat with polyunsaturated fat to reduce the incidence of cardiovascular disease [21, 22], and have increased the consumption of vegetable oils. The estimated per capita consumption of soybean oil, one of the major dietary sources of LNA in the US, has increased more than 1,000-fold from 1909 to 1999, increasing the availability of LNA from 2.8 to 7.2 en% [23]. We previously reported a robust positive correlation between the prevalence of obesity in the US and soybean oil, and with the other primary sources of LNA such as poultry and shortening [14]. Thus we believe that the high intake of LNA, of which soybean oil is the greatest contributor in the US diet, is a strong contributor to the obesity epidemic.
The endocannabinoids are endogenous lipid mediators formed from the pool of 20 carbon n-6 fatty acids present in membrane phospholipids (PL) [24]. The n-6 fatty acid arachidonic acid (ARA, 20:4n-6) in phospholipids is the precursors of the two best characterized endocannabinoids 2-AG and AEA. Since n-3 and n-6 fatty acids cannot be synthesized de novo the fatty acid composition in tissues is reflected by the dietary intake of these fatty acids [25]. An epidemiological report linked increasing intake of LNA over time to increased prevalence of obesity and postulated that ARA-induced elevation in 2-AG may have altered the energy balance towards obesity [26]. In a recent study we modeled the increase in human consumption of LNA and demonstrated that increasing dietary levels of LNA from 1 to 8 en%, elevated the levels of ARA–PL, 2-AG and AEA in liver and promoted obesity in mice fed high fat diets of 35 and 60 en% fat [14]. We showed that it was the n-3 and n-6 fat composition of the diets, rather than the total amount of fat that determined the obesogenic properties of the diets [14]. In the same study, we also reversed the obesogenic effect of high fat diets by isocalorically decreasing dietary LNA from 8 to 1 en% and attenuated the ARA-dependent excessive endocannabinoid activity [14]. Furthermore, we demonstrated that reducing the ARA–PL precursor pool by adding 1 en% eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3) to 8 en% LNA diets reversed both stimulation of endocannabinoid activity and the obesogenic effect of high fat diets [14]. Thus, the obesogenic potential of high fat diets can be reversed by selectively reducing the essential fatty acid precursors of endocannabinoids available only from dietary sources. As we have previously shown that 8 en% LNA elevate endocannabinoid levels and induce adiposity in high fat diets, we sought to investigate whether a low fat diet could be made obesogenic by similar elevations of dietary endocannabinoid precursors. Therefore we aimed to determine if selective isocaloric inclusion of 8 en% LNA in a low fat diet (12.5 en%) and medium fat diet (35 en%) resulted in endocannabinoid hyperactivity and obesity.
Materials and Methods
Ethical Statement
All animal experiments were approved by the National Animal Health Authorities (Norwegian approval identification 1973). Care and handling were in accordance with local institutional recommendations and rules, and no adverse events were observed. The animals were anesthetized with isoflurane to minimize suffering before decapitation.
Animals
Male mice (n = 36), 6 weeks of age (C57BL/6j, Taconic, Denmark) were randomly assigned to four experimental diets (n = 9) (Table 1) and housed individually. The animals were maintained on a 12:12 h light–dark cycle at 29 ± 1 °C. All animals were sacrificed at 22 weeks of age.
Water and Food
Food provided as pellets was available ad libitum for 16 weeks. The diets contained 12.5 en% fat (LFD) and 35 en% fat (MFD) and 18 and 22 en% protein from casein (LFD and MFD respectively). Dextrin was used as a neutral source of carbohydrate, and was used to compensate for the lower fat content in the LFDs. In order to model the increase in human consumption of LNA from 1 to 8 en% and isolate LNA and ALA as controlled variables different oils were mixed to obtain the specific fatty acid profile of the diets listed in Table 1. Food intake was measured every other day by weighing each food cup and spillage and subtracting the previously collected weight. Body weight was recorded once a week for all animals.
Endocannabinoids
Mice were sacrificed by decapitation and brain and liver were quickly snap-frozen in liquid nitrogen. The 2-AG and AEA were extracted and determined by gas chromatography/mass spectrometry/mass spectrometry (GC/MS/MS) as previously described [14].
Fatty Acid Profile
Liver, red blood cells (RBC) and adipose tissue lipids were extracted with chloroform: methanol (2:1 v/v) and phospholipids were separated from neutral lipids by solid phase extraction (SPE). Epididymal adipose tissue (eWAT) and liver lipids were evaporated to dryness and recovered in chloroform to a concentration of 50 mg/mL lipids. An aliquot of 200 μL (10 mg lipids) was applied to a SPE column (Isolute). RBC lipids were evaporated to dryness and recovered with three washings of 100 mL chloroform and deposited to the SPE column. Neutral lipids were eluted with 10 mL chloroform/methanol (98:2 v/v) and polar lipids were eluted with 20 mL methanol. The fatty acid composition in the phospholipid fraction of liver and RBC, and the neutral fraction of adipose tissue were analyzed by GC as previously described [27].
Blood Chemistry
Blood was collected in an Eppendorf tube from the bleeding carcass after decapitation and separated into erythrocytes and plasma. Plasma hormone levels were determined using commercially available ELISA kits in accordance with manufacturer’s instructions for insulin (DRG Diagnostics, Ultrasensitive ELISA, mouse, detection limit 0.025 μg/L, coefficient of variation within assay ~2 %, between assay ~4 %), leptin (ALPCO Immunoassays, Leptin (Mouse/Rat) ELISA, range 15–1,600 pg/mL, sensitivity 10 pg/mL, inter-assay and intra-assay variation coefficients <4.7 and <4.4 %, respectively) and adiponectin (ALPCO Immunoassays (Mouse) Total, HMW ELISA, range 0.125–8 ng/mL, sensitivity 0.032 ng/mL, inter-assay and intra-assay coefficient of variation ~3.5 %.).
Histology
Sections of adipose tissue were fixed in 4 % formaldehyde in 0,1 M phosphate buffer (PB) for 24 h, washed in PB, dehydrated in ethanol, and embedded in paraffin after clearing with xylene. 5 μm thick sections of the embedded tissue were stained with eosin and hematoxylin. Sections were visually examined using an Olympus BX 51 binocular microscope (Tokyo, Japan) fitted with a Nikon DS-Fi1 camera (Digital Sight DS-Fi1, Nikon, Japan). Adipocyte size was measured using the interactive measurement module of an image analysis system equipped with an Olympus microscope, a Nikon DS-Fi1 camera and NIS-elements software (Nikon, Japan). Two hundred adipocytes per tissue (n = 2) were randomly selected and their size was measured by drawing a horizontal line between the cell membranes.
Immunohistochemistry
Samples were processed in formaldehyde as described above. Then 5-μm sections were deparaffinized, rehydrated, and endogenous peroxide was inactivated (3 % hydrogen peroxide). To reduce non-specific staining the sections were incubated in heat-inactivated normal goat serum (10 %, 10 min). Sections were then incubated overnight at 4 °C with rat anti-mouse F4/80 (1:500; Serotec, Germany), subsequently washed in tris buffered saline (TBS, 3 times, 10 min) and incubated with HRP-conjugated goat anti-rat IgG (1:250; Serotec, Germany) for 2 h. After washing in TBS (3 × 10 min) specific binding was visualized using diamino benzidine. The immunohistochemistry was performed and examined by a scientist masked to the experimental conditions.
Statistics
All data are analyzed using StatSoft, Inc. (2009) STATISTICA (data analysis software system), version 9.0. Data were analyzed for homogeneity of variance (Levene’s test) and one-way ANOVA, with Fisher LSD post hoc test when p < 0.05. Data are presented as mean ± standard error of the mean (SEM).
Results
Dietary LNA Increased Tissue Arachidonic Acid and Endocannabinoid Levels
Diets containing 8 en%, reflecting current US intake, resulted in significantly higher amounts of LNA and ARA in RBC–PL (Table 2), liver–PL (Table 3), and eWAT (Table 4) compared to diets containing 1 en% LNA. Consequently, elevating LNA from 1 to 8 en% in both LFD and MFD significantly increased 2-AG and AEA in liver (Fig. 1a, b). The brain is less influenced by dietary manipulation and we found no differences in endocannabinoid levels in the cerebral cortex (Fig. 1c, d). Elevating dietary LNA from 1 to 8 en% significantly reduced EPA–PL in both RBC (Table 2) and liver (Table 3). The concentration of DHA–PL was increased in RBC of mice fed LFD and MFD containing 1 en% LNA (Table 2), but only in livers of mice fed LFD 1 en% (Table 3). Thus, increasing dietary LNA from 1 to 8 en% decreased the n-3 index in RBC-PL from 9 to 5 (Table 2). Mice fed 8 en% LNA had significantly higher EPA and DHA in eWAT than mice fed 1 en% LNA in both LFD and MFD (Table 4).
Selective elevation of dietary LNA elevates liver endocannabinoids in mice fed low fat diets (LFD) of 12.5 en% fat (light gray) and medium fat diets (MFD) of 35 en% fat (dark gray). Increasing dietary LNA from 1 en% (open bars) to 8 en% (hatched bars) in both LFD and MFD elevated (a) liver 2-AG and (b) AEA, an increase that was not observed in the cerebral cortex (c, d). Different superscript letters indicate a statistical significance p < 0.05 by ANOVA, n = 8–9
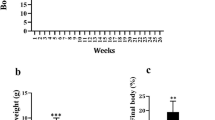
Dietary LNA Increased Weight Gain and Body Weight in Low Fat Diets
Food intake was not affected by the dietary treatments (Table 5). As expected, mice fed MFDs gained more body weight and tended to accumulate more adipose tissue than mice fed an LFD when dietary LNA was kept low at 1 en% (Fig. 2, Table 5). Of note, when dietary LNA was elevated from 1 to 8 en% in the low fat diet, feed efficiency increased resulting in a similar weight gain and body weight as the MFDs (Fig. 2, Table 5). Muscle weight did not differ between the groups (data not shown), indicating that differences in body weight was due to accumulation of adipose tissue and not lean mass.
Dietary LNA Elevated Plasma Leptin Levels
The LFD and MFD containing 8 en% LNA significantly elevated plasma leptin concentrations compared to 1 en% LNA diets (Table 5). Mice fed LFDs of 1 and 8 en% LNA had similar plasma adiponectin levels per gram fat as mice fed 8 en% LNA in the MFD and significantly higher body fat levels than mice fed 1 en% in the MFD (Table 5). Plasma insulin concentrations were not affected by the dietary treatments (Table 5).
Dietary LNA Increased Adipose Accumulation and Adipocyte Size
The accumulation of white adipose tissue (WAT) and the adiposity index [(subcutaneous + retroperitoneal + inguinal fat pads)/eviscerated body weight × 100] was similar in mice fed 8 en% LNA in an LFD and mice fed the MFDs (Table 5). Mice fed 1 en% LNA in the LFD had significantly less adipose tissue and lower adiposity index than mice fed 8 en% LNA in a MFD (Table 5). We performed immunohistochemical analysis of adipose tissue by staining for the MI macrophage marker F4/80. We only found macrophage infiltration in eWAT of mice fed 8 en% LNA diets inducing F4/80 positive macrophages forming crown-like structures around adipocytes (Fig. 3a). No macrophage infiltration was found in iWAT (data not shown). These results indicate that the composition of dietary fat is substantially more important than the total amount of fat in determining adipose tissue accumulation and macrophage infiltration (Fig. 3a, b).
Discussion
We have previously shown that modeling the increase in human consumption of dietary LNA from 1 to 8 en% LNA during the last century [23] caused excessive endocannabinoid levels and also induces obesity in mice fed diets of 35 and 60 en% fat [14]. To our knowledge, we demonstrate here for the first time that an LFD can be made obesogenic by inclusion of 8 en% LNA, subsequently stimulating excessive endocannabinoid activity in the liver and weight gain. In the present study we report that isocalorically increasing dietary LNA from 1 to 8 en% elevated tissue levels ARA–PL, liver 2-AG and AEA regardless of changes in total dietary fat, and increased feed efficiency, caused higher weight gain and elevated plasma leptin in mice fed the LFD. We also observed a tendency of increased cell size and macrophage infiltration in eWAT in mice fed 8 en% LNA.
The percent of n-6 in HUFA can be used as an indicator of disease risk as it models the relative amounts of tissue ARA–PL available as precursors for eicosanoid derivatives of ARA [28]. We used the Lands equation [25] to predict tissue ARA–PL composition as the % n-6 HUFA from dietary intakes of n-6 and n-3 PUFA to model availability of endocannabinoid precursors with good concurrence between calculated and experimental values. Here we modeled the increase in US dietary intakes of LNA from 1 to 8 en% [23] in our animal diets, and showed in mice, that elevating dietary LNA to 8 en% increased ARA–PL in liver, RBC and adipose tissue to levels currently found in Americans [29], and induced elevations in liver 2-AG and AEA. Elevating endocannabinoid levels by dietary LNA to alter the availability of ARA in the phospholipid precursor pool is consistent with several studies where dietary fatty acids alter endocannabinoid levels [30–36], and our previous work where we selectively altered LNA and raised dietary omega-3 HUFA both in mice and salmon [14, 15]. We did not observe any differences in food intake or endocannabinoid levels in the cerebral cortex, suggesting that the effects of increased endocannabinoid levels were caused by effects in peripheral tissue. The adult brain is less susceptible to dietary manipulations than the young brain [37, 38]. The lower sensitivity of dietary LNA on brain endocannabinoid levels compared to peripheral tissues confirm that brain lipids levels are less influenced by changes in dietary fatty acids and that this may be reflected also in the endocannabinoids [39, 40].
Dietary LNA of 8 en%, independent of dietary fat content, significantly elevated plasma leptin levels compared to 1 en% LNA. Plasma adiponectin levels were less affected by dietary LNA, suggesting that leptin is a more sensitive marker of early obesity development. Mice fed an LFD had similar plasma concentrations of adiponectin per gram adipose tissue despite higher WAT and adiposity in mice fed 8 en% LNA compared to 1 en% LNA. This result suggests that mice fed a high LNA content secrete less adiponectin per gram fat, in line with the obesity-prone phenotype of the 8 en% LNA diet.
We find that the LFD containing 8 en% LNA significantly increased feed efficiency compared to the 1 en% LNA diet causing the animals to gain more weight per calorie consumed. The increased feed efficiency and weight gain in mice fed 8 en% LNA in the LFD resulted in a similar body weight as mice fed 35 en% fat, significantly higher than mice fed 1 en% LNA in an LFD. Dietary LNA of 1 en% has been found to reverse the obesogenic properties of a high fat diet [14]. In line with previous studies, our findings in the LFD support the notion that weight gain involves mechanisms that depend more on the composition of dietary fat than the total amount of fat in the diet [12, 30, 41–43]. Increased intake of LNA has been linked to increased prevalence of obesity [26, 41] and the primary dietary sources of LNA; soybean oil, poultry and shortening were positively correlated to increasing rates of obesity occurring in the US during the 20th century [14]. Replacing saturated fats with LNA-rich vegetable oil increased body weight in veterans [44] and ARA levels in adipose tissue were positively associated with BMI in children [45]. In animals, vegetable oils rich in LNA (usually soybean oil; 55 % LNA, and safflower oil; 75 % LNA) elevated food intake [14, 15, 46], induced weight gain [14, 15, 30, 41, 42, 47] and increased lipogenic enzyme activity in the liver [10, 48]. Our findings imply that low fat diets could be made more effective in reducing adiposity if LNA were lowered to near 1 en%. Indeed, total dietary fat intake may not need to be lowered if LNA is selectively lowered.
In contrast to what we previously reported [14], 1 en% LNA in the MFD did not prevent weight gain and adiposity compared to MFD 8 en% LNA. In the present study animals were fed the experimental diets from 6 weeks of age, whereas in our previous study animals were exposed to the dietary treatments from the last week of pregnancy to 17 weeks of age [14]. D’Asti et al. [49] demonstrated how quantity and quality of maternal dietary fat during the perinatal period directly influences neonatal metabolism, fatty acid composition in phospholipids and sensitivity to endocannabinoid system manipulation. Massiera et al. [50] found a gradual transgenerational increase in adiposity in mice fed a “western-like” diet of 35 en% fat containing 18 en% LA for four generations. Thus the adipogenic effect of LNA appears to be higher when prenatally exposed [14]. Additionally, in the present study, mice were fed a standard NIH #31M diet based on soybean oil prior to arrival at our facility (Taconic, Denmark). Calculations by the Lands equation [25] show that the NIH #31M diet results in 72 % n-6 HUFA in tissue phospholipids, identical to our diets containing 8 en% LNA. Metabolic changes such as induced lipogenesis and deposition of lipid droplets in response to increased endocannabinoid activity have been shown to occur before onset of obesity [10, 12, 51]. It is possible that an already elevated endocannabinoid activity present in the animals upon arrival to our facility may have overridden the anti-adipogenic properties of the 1 en% LNA diet, when the MFD contained abundant carbohydrates [42]. The latter might be of importance as it has been demonstrated that the obesogenic potential of diets rich in both n-6 and n-3 PUFA is elevated by increasing the levels of dietary carbohydrates, and thereby raising the insulin/glucagon ratio translated into reduced cAMP signaling [42, 52]. cAMP signaling has been demonstrated to play a pivotal role in controlling the production of both pro- and anti-adipogenic prostaglandins both in vivo and in vitro [42]. Collectively these results illustrate the importance of the type of fat and carbohydrates in the background diet, and how these can influence the obesogenic properties of LNA. Mice fed an LFD of 8 en% LNA started to gain considerably more weight than mice fed 1 en% LNA after 8 weeks of feeding. Thus a longer feeding period may be necessary to wash out excessive tissue ARA caused by the NIH #31M diet, before a beneficial effect of a low LNA diet (1 en%) may occur. Although body weight did not differ, elevated liver 2-AG and AEA, larger adipocytes, higher macrophage accumulation in WAT and elevated plasma leptin levels in mice fed 8 en% may suggest a more obesity prone phenotype and a higher risk of developing obesity and metabolic complications associated with obesity compared to mice fed same amount of fat but with only 1 en% LNA.
Dietary LNA increase macrophage infiltration and cell size in adipose tissue. (a) Immunostaining with F4/80 showed more crown-like structures indicating macrophage infiltration (indicated by arrows) in eWAT of mice fed 8 en% LNA compared to mice fed 1 en% LNA. (b) HE staining of iWAT. Mice fed 8 en% LNA displayed larger adipocyte cell size in (a) eWAT and (b) iWAT in both low fat (LF) and medium fat diets (MF) compared to mice fed 1 en% LNA. Data are presented as min to max, line at median, n = 2. Scale bar 100 μm
One of the major limitations when working with dietary fat is to relate the observed differences to one fatty acid. There are no inert oils which can be added to a diet to target one single fatty acid to be different in a diet. We did extensive analyses of various fats and oils available on the market to establish the fatty acid profile of the products and carefully mixed several oils to isolate LNA as controlled variable via isocaloric replacement of saturated fat (in the medium fat diet). It was not possible to obtain similar content of MUFA in the low fat diets since the low fat diet contained 8 en% fat as LA of the total of 12.5 en% fat. Although our diets contained different amounts of SFA and MUFA, SFA were covariable in the LFD and MUFA were covariable in the MFD, thus strengthening our conclusion that LNA is the major dietary fatty acid affecting endocannabinoid levels and induces weight gain. If there were other fatty acids (MUFA or SFA) that strongly influenced endocannabinoid levels and adiposity we would expect the differences between the dietary treatments to be less distinct. Another limitation with our study is the absence of endocannabinoid measurements in adipose tissue, making it difficult to conclude if the observed elevation of endocannabinoid levels in the liver is a consequence rather than the cause of obesity. In order to establish a cause–effect relationship between a diet induced elevation in endocannabinoid levels and the observed obese phenotype in mice fed the LFD of 8 en% LNA a follow-up study should be carried out using a CB1 antagonist.
Reducing dietary LNA from 8 to 1 en% with a concomitant decrease in ALA from 1 to 0.3 en% significantly increased EPA–PL concentrations in RBC (three-fold) and liver, increasing the n-3 index from 5 to 8 and 9 in MFD and LFD respectively. We have previously reported that reducing LNA from 8 to 1 en% increased EPA and reduced ARA in liver and RBC similarly to supplementing an 8 en% LNA diet with 1 en% EPA + DHA [14]. Consistent with previous reports [53–58], our results indicate that the elongation and desaturation of ALA to EPA and DHA are considerably more effective when dietary LNA is 1 en%. Hence to improve tissue EPA and DHA concentrations, reduce ARA–PL and consequently decrease endocannabinoid production, emphasis should be on lowering dietary LNA in addition to dietary supplementation with EPA and DHA. In conclusion, a dietary approach by reducing substrate availability for endocannabinoid synthesis provides a safe and preventative alternative to decrease hyperactivity of the endocannabinoid system, and consequently decrease or prevent obesity.
Abbreviations
- 2-AG:
-
Arachidonoylglycerol
- ARA:
-
Arachidonic acid
- AEA:
-
Anandamide
- CB:
-
Cannabinoid receptor
- DHA:
-
Docosahexaenoic acid
- en%:
-
Percent of energy
- EPA:
-
Eicosapentaenoic acid
- eWAT:
-
Epididymal white adipose tissue
- HUFA:
-
Highly unsaturated fatty acid(s) (fatty acids of >20 carbon length)
- iWAT:
-
Inguinal white adipose tissue
- LFD:
-
Low fat diet
- LNA:
-
Linoleic acid
- MFD:
-
Medium fat diet
- MUFA:
-
Monounsaturated fatty acid(s)
- PL:
-
Phospholipids
- RBC:
-
Red blood cells
- SFA:
-
Saturated fatty acid(s)
References
Devane WA, Axelrod J (1994) Enzymatic synthesis of anandamide, an endogenous ligand for the cannabinoid receptor, by brain membranes. Proc Natl Acad Sci USA 91:6698–6701
Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA et al (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258:1946–1949
Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE et al (1995) Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 50:83–90
Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A et al (1995) 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun 215:89–97
Devane WA, Dysarz FA 3rd, Johnson MR, Melvin LS, Howlett AC (1988) Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol 34:605–613
Munro S, Thomas KL, Abu-Shaar M (1993) Molecular characterization of a peripheral receptor for cannabinoids. Nature 365:61–65
Cota D, Marsicano G, Tschop M, Grubler Y, Flachskamm C et al (2003) The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest 112:423–431
Poirier B, Bidouard JP, Cadrouvele C, Marniquet X, Staels B et al (2005) The anti-obesity effect of rimonabant is associated with an improved serum lipid profile. Diabetes Obes Metab 7:65–72
Ravinet Trillou C, Arnone M, Delgorge C, Gonalons N, Keane P et al (2003) Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol 284:R345–R353
Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S et al (2005) Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest 115:1298–1305
Bensaid M, Gary-Bobo M, Esclangon A, Maffrand JP, Le Fur G et al (2003) The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol 63:908–914
Matias I, Petrosino S, Racioppi A, Capasso R, Izzo AA et al (2008) Dysregulation of peripheral endocannabinoid levels in hyperglycemia and obesity: effect of high fat diets. Mol Cell Endocrinol 286:S66–S78
Starowicz KM, Cristino L, Matias I, Capasso R, Racioppi A et al (2008) Endocannabinoid dysregulation in the pancreas and adipose tissue of mice fed with a high-fat diet. Obesity (Silver Spring) 16:553–565
Alvheim AR, Malde MK, Osei-Hyiaman D, Hong Lin Y, Pawlosky R et al (2012) Dietary linoleic acid elevates endogenous 2-AG and anandamide and induces obesity. Obesity (Silver Spring) 20:1984–1994
Alvheim A, Torstensen B, Hong Lin Y, Lillefoss H, Lock E-J et al (2012) Dietary linoleic acid elevates endogenous 2-arachidonoylglycerol and anandamide in Atlantic salmon (Salmo salar L.) and mice, and induces weight gain and inflammation in mice. Br J Nutr 109(8):1508–1517. doi:10.1017/S0007114512003364
Osei-Hyiaman D, Harvey-White J, Batkai S, Kunos G (2006) The role of the endocannabinoid system in the control of energy homeostasis. Int J Obes (Lond) 30(Suppl 1):S33–S38
Despres JP (2007) The endocannabinoid system: a new target for the regulation of energy balance and metabolism. Crit Pathw Cardiol 6:46–50
Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S (2005) Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 365:1389–1397
Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A (2007) Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet 370:1706–1713
Christopoulou FD, Kiortsis DN (2011) An overview of the metabolic effects of rimonabant in randomized controlled trials: potential for other cannabinoid 1 receptor blockers in obesity. J Clin Pharm Ther 36:10–18
Harris WS, Mozaffarian D, Rimm E, Kris-Etherton P, Rudel LL et al (2009) Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation 119:902–907
Mozaffarian D, Micha R, Wallace S (2010) Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med 7:e1000252
Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR (2011) Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr 93(5):950–962
Wang J, Ueda N (2009) Biology of endocannabinoid synthesis system. Prostaglandins Other Lipid Mediat 89:112–119
Lands WE, Libelt B, Morris A, Kramer NC, Prewitt TE et al (1992) Maintenance of lower proportions of (n-6) eicosanoid precursors in phospholipids of human plasma in response to added dietary (n-3) fatty acids. Biochim Biophys Acta 1180:147–162
Ailhaud G, Massiera F, Weill P, Legrand P, Alessandri J-M et al (2006) Temporal changes in dietary fats: role of n-6 polyunsaturated fatty acids in excessive adipose tissue development and relationship to obesity. Prog Lipid Res 45:203–236
Torstensen BE, Nanton DA, Olsvik PA, Sundvold H, Stubhaug I (2009) Gene expression of fatty acid-binding proteins, fatty acid transport proteins (cd36 and FATP) and beta-oxidation-related genes in Atlantic salmon (Salmo salar L.) fed fish oil or vegetable oil. Aquac Nutr 15:440–451
Lands B (2009) Measuring blood fatty acids as a surrogate indicator for coronary heart disease risk in population studies. World Rev Nutr Diet 100:22–34
Stark KD (2008) The percentage of n-3 highly unsaturated fatty acids in total HUFA as a biomarker for omega-3 fatty acid status in tissues. Lipids 43:45–53
Matias I, Carta G, Murru E, Petrosino S, Banni S et al (2008) Effect of polyunsaturated fatty acids on endocannabinoid and N-acyl-ethanolamine levels in mouse adipocytes. Biochim Biophys Acta 1781:52–60
Artmann A, Petersen G, Hellgren LI, Boberg J, Skonberg C et al (2008) Influence of dietary fatty acids on endocannabinoid and N-acyl ethanolamine levels in rat brain, liver and small intestine. Biochim Biophys Acta 1781:200–212
Berger A, Crozier G, Bisogno T, Cavaliere P, Innis S et al (2001) Anandamide and diet: inclusion of dietary arachidonate and docosahexaenoate leads to increased brain levels of the corresponding N-acylethanolamines in piglets. Proc Natl Acad Sci USA 98:6402–6406
Watanabe S, Doshi M, Hamazaki T (2003) n-3 Polyunsaturated fatty acid (PUFA) deficiency elevates and n-3 PUFA enrichment reduces brain 2-arachidonoylglycerol level in mice. Prostaglandins Leukot Essent Fatty Acids 69:51–59
Piscitelli F, Carta G, Bisogno T, Murru E, Cordeddu L et al (2011) Effect of dietary krill oil supplementation on the endocannabinoidome of metabolically relevant tissues from high-fat-fed mice. Nutr Metab (Lond) 8:51
Di Marzo V, Griinari M, Carta G, Murru E, Ligresti A et al (2010) Dietary krill oil increases docosahexaenoic acid and reduces 2-arachidonoylglycerol but not N-acyl ethanolamine levels in the brain of obese Zucker rats. Int Dairy J 20:231–235
Wood JT, Williams JS, Pandarinathan L, Janero DR, Lammi-Keefe CJ et al (2010) Dietary docosahexaenoic acid supplementation alters select physiological endocannabinoid-system metabolites in brain and plasma. J Lipid Res 51:1416–1423
Rapoport SI, Rao JS, Igarashi M (2007) Brain metabolism of nutritionally essential polyunsaturated fatty acids depends on both the diet and the liver. Prostaglandins Leukot Essent Fatty Acids 77:251–261
Demar JC, DiMartino C, Baca AW, Lefkowitz W, Salem N (2008) Effect of dietary docosahexaenoic acid on biosynthesis of docosahexaenoic acid from alpha-linolenic acid in young rats. J Lipid Res 49:1963–1980
Wood JT, Williams JS, Pandarinathan L, Janero DR, Lammi-Keefe CJ et al (2010) Dietary docosahexaenoic acid supplementation alters select physiological endocannabinoid-system metabolites in brain and plasma. J Lipid Res 51:1416–1423
Di Marzo V, Griinari M, Carta G, Murru E, Ligresti A et al (2010) Dietary krill oil increases docosahexaenoic acid and reduces 2-arachidonoylglycerol but not N-acylethanolamine levels in the brain of obese Zucker rats. Int Dairy J 20:231–235
Massiera F, Saint-Marc P, Seydoux J, Murata T, Kobayashi T et al (2003) Arachidonic acid and prostacyclin signaling promote adipose tissue development: a human health concern? J Lipid Res 44:271–279
Madsen L, Pedersen LM, Liaset B, Ma T, Petersen RK et al (2008) cAMP-dependent signaling regulates the adipogenic effect of n-6 polyunsaturated fatty acids. J Biol Chem 283:7196–7205
Allhaud G, Guesnet P, Cunnane SC (2008) An emerging risk factor for obesity: does disequilibrium of polyunsaturated fatty acid metabolism contribute to excessive adipose tissue development? Br J Nutr 100:461–470
Dayton S, Hashimoto S, Dixon W, Pearce ML (1966) Composition of lipids in human serum and adipose tissue during prolonged feeding of a diet high in unsaturated fat. J Lipid Res 7:103–111
Savva SC, Chadjigeorgiou C, Hatzis C, Kyriakakis M, Tsimbinos G et al (2004) Association of adipose tissue arachidonic acid content with BMI and overweight status in children from Cyprus and Crete. Br J Nutr 91:643–649
Takahashi Y, Ide T (2000) Dietary n-3 fatty acids affect mRNA level of brown adipose tissue uncoupling protein 1, and white adipose tissue leptin and glucose transporter 4 in the rat. Br J Nutr 84:175–184
Ikemoto S, Takahashi M, Tsunoda N, Maruyama K, Itakura H et al (1996) High-fat diet-induced hyperglycemia and obesity in mice: differential effects of dietary oils. Metabolism 45:1539–1546
Cleary MP, Phillips FC, Morton RA (1999) Genotype and diet effects in lean and obese Zucker rats fed either safflower or coconut oil diets. Proc Soc Exp Biol Med 220:153–161
D’Asti E, Long H, Tremblay-Mercier J, Grajzer M, Cunnane SC et al (2010) Maternal dietary fat determines metabolic profile and the magnitude of endocannabinoid inhibition of the stress response in neonatal rat offspring. Endocrinology 151:1685–1694
Massiera F, Barbry P, Guesnet P, Joly A, Luquet S et al (2010) A western-like fat diet is sufficient to induce a gradual enhancement in fat mass over generations. J Lipid Res 51:2352–2361
Xu HY, Barnes GT, Yang Q, Tan Q, Yang DS et al (2003) Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112:1821–1830
Ma T, Liaset B, Hao Q, Petersen RK, Fjaere E et al (2011) Sucrose counteracts the anti-inflammatory effect of fish oil in adipose tissue and increases obesity development in mice. PLoS ONE 6:e21647
Liou YA, King DJ, Zibrik D, Innis SM (2007) Decreasing linoleic acid with constant alpha-linolenic acid in dietary fats increases (n-3) eicosapentaenoic acid in plasma phospholipids in healthy men. J Nutr 137:945–952
Munakata M, Nishikawa M, Togashi N, Nio E, Kobayashi Y et al (2009) The nutrient formula containing eicosapentaenoic acid and docosahexaenoic acid benefits the fatty acid status of patients receiving long-term enteral nutrition. Tohoku J Exp Med 217:23–28
Novak EM, Dyer RA, Innis SM (2008) High dietary omega-6 fatty acids contribute to reduced docosahexaenoic acid in the developing brain and inhibit secondary neurite growth. Brain Res 1237:136–145
Bourre JM, Bonneil M, Dumont O, Piciotti M, Calaf R et al (1990) Effect of increasing amounts of dietary fish oil on brain and liver fatty composition. Biochim Biophys Acta 1043:149–152
Mohrhauer H, Holman RT (1963) Effect of linolenic acid upon the metabolism of linoleic acid. J Nutr 81:67–74
Clark KJ, Makrides M, Neumann MA, Gibson RA (1992) Determination of the optimal ratio of linoleic acid to alpha-linolenic acid in infant formulas. J Pediatr 120:S151–S158
Harris WS, von Schacky C (2004) The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med 39:212–220
Acknowledgments
This study was funded by the National Institute of Nutrition and Seafood Research (NIFES), Bergen, Norway, Intramural Research program of the National Institute on Alcohol Abuse and Alcoholism, NIH, USA, and the Research Council of Norway 186908/l10.
Conflict of interest
The authors declare no conflicting interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Alvheim, A.R., Torstensen, B.E., Lin, Y.H. et al. Dietary Linoleic Acid Elevates the Endocannabinoids 2-AG and Anandamide and Promotes Weight Gain in Mice Fed a Low Fat Diet. Lipids 49, 59–69 (2014). https://doi.org/10.1007/s11745-013-3842-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-013-3842-y