Abstract
Nutritional influences on cardiovascular disease operate throughout life. Studies in both experimental animals and humans have suggested that changes in the peri- and early post-natal nutrition can affect the development of the various components of the metabolic syndrome in adult life. This has lead to the hypothesis that n-3 fatty acid supplementation in pregnancy may have a beneficial effect on lipid profile in the offspring. The aim of the present study was to investigate the effect of supplementation with n-3 fatty acids during the third trimester of pregnancy on lipids and lipoproteins in the 19-year-old offspring. The study was based on the follow-up of a randomized controlled trial from 1990 where 533 pregnant women were randomized to fish oil (n = 266), olive oil (n = 136) or no oil (n = 131). In 2009, the offspring were invited to a physical examination including blood sampling. A total of 243 of the offspring participated. Lipid values did not differ between the fish oil and olive oil groups. The relative adjusted difference (95% confidence intervals) in lipid concentrations was −3% (−11; 7) for LDL cholesterol, 3% (−3; 10) for HDL cholesterol, −1% (−6; 5) for total cholesterol,−4% (−16; 10) for TAG concentrations, 2%(−2; 7) for apolipoprotein A1, −1% (−9; 7) for apolipoprotein B and 3% (−7; 15) in relative abundance of small dense LDL. In conclusion, there was no effect of fish oil supplementation during the third trimester of pregnancy on offspring plasma lipids and lipoproteins in adolescence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Development of cardiovascular disease (CVD) is a lifelong process [1, 2]. This has led to the hypothesis that exposures early in life may play a role for the later development of CVD. High blood pressure, obesity, atherosclerosis and diabetes develop over time, and risk factors may be detectable early in life [2–6]. Prevention may therefore be possible by targeting factors leading to CVD early in life.
A lipid profile, with high plasma levels of LDL-cholesterol, triacylglycerols (TAG) and apolipoprotein B100 (Apo-B) and a low concentration of HDL-cholesterol and apolipoprotein A1 (Apo-A), increase the risk of atherosclerosis and CVD [7, 8]. Also, plasma LDL particles are comprised of different sub-fractions, differing in chemical composition, size and density, and studies have suggested that particularly the fraction of small dense LDL particles (sdLDL) is associated with CVD [9, 10]. sdLDL is strongly correlated with plasma TAG, and generally the strong correlation between the different lipid fractions makes it difficult to identify the contribution of the separate fractions to the risk of CVD [7, 8, 11].
Fish consumption and supplementation with n-3 polyunsaturated fatty acids (PUFA) in adulthood have been associated with slightly increased levels of HDL cholesterol, lower TAG concentration, and both a lower relative abundance and particle number of sdLDL [12, 13]. Also, fish oil supplementation during infancy has been found to be associated with a decrease in plasma TAG concentration and an increase in the concentration of plasma LDL and total cholesterol [14, 15]. It is, however, not known whether these effects of n-3 PUFA found early in life can track and program the future lipid profile of the child [16].
Breast feeding has also been shown to increase cholesterol concentration in infancy [17]. These short-term effects of breast feeding are, translated into a long-term decrease in total cholesterol in adulthood [17]. This effect is thought to be caused by the relatively high cholesterol concentration of breast milk, leading to a decrease in the endogenous production of cholesterol. However, breast milk also contains high concentrations of n-3 PUFA, which could potentially influence lipid metabolism. To our knowledge, only one study has looked into the long-term effect of fish oil supplementation during early life on the lipid profile in adulthood [18]. In this study, no association between increased dietary intake of n-3 PUFA during the first 5 years of life and lipid profile at the age of 8 years, was found.
We investigated the hypothesis that supplementation with long chain marine n-3 PUFA during fetal life has an impact on the plasma lipid profile in adolescence. This was done by studying offspring from a randomized controlled trial conducted in 1990, where pregnant women were randomized to fish oil, olive oil or no oil [19]. No association between fish oil supplementation during pregnancy and blood lipid profile in the offspring was found.
Methods
The aim of the original study was to investigate the effect of fish oil supplementation on gestational length. The recruitment and randomization of the original study population has previously been described in detail [19].
Briefly, 533 women in gestational week 30 who attended the Midwife Centre in Aarhus, Denmark, were randomized to four 1 g fish oil capsules (FO) (Pikasol: 32% eicosapentaenoic acid and 23% docosahexaenoic acid, corresponding to approximately 2.7 g marine n-3 PUFA) per day (n = 266), four similar capsules with olive oil (OO) (n = 136) or no capsules (NO) (n = 131) in 1990. The women receiving oil were blinded for study interventions, and the capsules and boxes looked identical. The 533 enrolled and randomized women represented 61% of eligible women.
The offspring from the abovementioned randomized controlled trial constituted the study population in the present study. At the time of the study, the offspring were aged between 18 and 19 years. A total of 517 (97%) mother and child pairs were alive and living in Denmark.
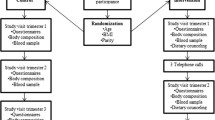
All the mothers were contacted by mail and they invited their children to complete a self-administered web-based questionnaire concerning anthropometric measures, health and lifestyle. The offspring were also asked whether they wanted to receive an invitation for a physical examination. Those accepting and those who did not respond were all invited to the physical examination. A total of 382 filled out the questionnaire and 243 participated in the clinical examination (Fig. 1).
At the physical examination, a fasting venous blood sample was drawn, centrifuged and frozen at −80 °C.
Serum TAG and cholesterol fractions (Total, LDL, HDL) were measured according to standard methods on a Modular P from Roche Diagnostics, Basel, Switzerland. Apo-B was measured using antibody from DAKO, Glostrup, Denmark, on an Advia 1650 from Bayer Diagnostics, NY, USA. Interserial variation was 5.5%. Apo-A was measured using antibody from DAKO, Glostrup, Denmark, on an Advia 1650 from Bayer Diagnostics, NY, USA. Interserial variation was 5%.
For the sdLDL analysis, blood, anticoagulated with K3-EDTA 1.6 mg/ml, was centrifuged and plasma stored until analysis. Plasma, adjusted to 1.067 g/L with 60% iodixanol from Optiprep, Axis-Schield PoC As, Oslo, Norway, was prestained with Coomassie blue, under-layered beneath 9% iodixanol and subjected to ultracentrifugation (2½ h, 65.000 rpm 16 °C (341.000 g) in a near vertical rotor (Beckmann NVT65). A digital photograph of LDL subclass profiles was analyzed using Total Lab 1D gel-scan software (Pharmacis, UK). LDL subclass phenotypes A, B and I (Intermediate) were characterized according to the density and to the area under the curve of B (%AUC B) (sd-LDL) as follows: A: AUC B <40%, I: AUC B 40-50%, B: AUC B >50%. The method has been described in detail previously [20].
Covariates
Information on the mother was collected from the interview and questionnaire during pregnancy. The pregnant women filled out a simple food frequency questionnaire in order to assess their baseline fish consumption, and three categories were defined according to fish consumption. Characteristics of the children were collected from the questionnaire at 18–19 years of age. For this reason, we only had information regarding covariates for the non-participating children who filled out the questionnaire. Information on sex, gestational age and birth weight were collected from medical records.
This study was conducted according to the guidelines laid down in the declaration of Helsinki and all procedures involving human subjects were approved by the local ethics committee (case no.: M-ÅA 20060182) and the Danish Data Protection Agency (journal no.: 2006-41-6257). Written informed consent was obtained from all subjects.
Statistics
The OO group was used as the reference in the study for the following reasons: (1) the original study showed that blinding worked well in the OO group, indicating that this group was unlikely to have increased their fish consumption during the trial period. (2) It seems reasonable to assume that OO in the supplemented amounts was inert. The results from the NO group are, however, also presented in the tables.
The distribution of biochemical variables was generally skewed, and therefore log transformation was applied to normalize the distribution. Geometric means and inter-quartile ranges are presented.
Chi-square tests and Student’s t-tests were used to compare categorical and normally distributed continuous covariates, respectively, between participants and non-participants as well as between the FO and OO groups with two-sided p-values <0.05 considered statistically significant. For covariates that were not normally distributed or did not have the same variance in the two groups, Wilcoxon rank sum test was used to test for differences between groups.
Differential programming effects may be found in boys and girls, and therefore the analyses used to estimate the association between fish oil and later lipid profile were initially stratified by sex, using ANOVA. Since the associations were similar in males and females, multiple linear regression modeling adjusting for sex was used to estimate the association. Also, since supplementation with fish oil would be expected to have the largest effect among pregnant women with a low baseline fish intake, the multiple linear regression analyses were also done by only including participants of mothers with a low baseline fish intake (n = 46). All associations are reported as percentage difference, since they were all analyzed on the log-scale.
In addition to analyzing the effect on the relative abundance of sdLDL (% of total number of LDL particles) using multiple linear regression, also the effect on the prevalence of LDL subclass B (prevalence of sdLDL >50%) was estimated by logistic regression, adjusting for sex.
Results
Participants
Characteristics of those participating in the clinical examination and the non-participants are given in Table 1. The participants differed from the non-participants with regard to the mother’s age during pregnancy, sex and birth weight. Also, the participation rates differed between randomization groups, being lower in the FO group (41%) compared with the OO group (53%). A number of women in the FO group experienced side effects such as gastric reflux during supplementation. When invited to the follow-up, a few mothers reported that they did not think it was relevant for their child to participate since they did not comply with the original study protocol, due to side effects. The women were contacted in writing and the importance of participating was explained, irrespective of compliance. However, among those participating in the clinical examination from the FO group, 1.9% of the mothers took less than 75% of the daily dosage as opposed to 8.6% among non-participants. In the OO group, 2.8% of those participating in the follow-up and 3.2% of non-participants took less than 75% of the daily dosage. There was no information on compliance for 14.6 and 20.9% of the women receiving FO and OO, respectively.
A description of the participants in the three randomization groups is given in Table 2. The three groups were similar with respect to most covariates. The only difference reaching statistical significance was the smoking status in the offspring, with smoking being more prevalent among participants from the OO group compared to the other two groups.
The participants in the study were generally healthy. The median (interquartile range) was 4.0 (3.6; 4.7) mmol/l for total cholesterol, 1.3 (1.1; 1.6) mmol/l for HDL cholesterol, 2.3 (1.8; 2.7) mmol/L for LDL cholesterol and 0.9 (0.6; 1.2) mmol/L for TAG. In light of the recommended lipid levels in healthy subjects from international guidelines a total of 14% had a total cholesterol concentration above 5 mmol/L, 9% an HDL cholesterol concentration less than 1 mmol/L, 18% a LDL cholesterol concentration above 3 mmol/L and only 3% had a TAG concentration above 2 mmol/L.
Fish oil supplementation during pregnancy was not associated with cholesterol concentrations (total cholesterol, LDL, and HDL), TAG concentration or Apo-A1 and Apo-B in the offspring (Table 3). Also, no association was found with relative abundance of sdLDL. However, when restricting the analyses to participants of mothers with a low baseline fish intake, there was a tendency towards a healthier lipid and lipoprotein profile in participants from the FO group compared with the OO group (Table 4). Particularly, TAG, Apo-B and LDL cholesterol tended to be lower in the FO group, but none of the differences were statistically significant.
The distribution of LDL subclass phenotypes A, B and I (Intermediate) in the three randomization groups, stratified by sex, is shown in Fig. 2. There was a tendency towards a lower prevalence of phenotype B and larger prevalence of phenotype I in the FO and NO groups compared to the OO group. However, the difference did not reach statistical significance. Sex was significantly associated with LDL phenotype, with phenotype B being more prevalent among males.
Distribution of LDL subclass phenotypes A, B and I (Intermediate) in the three randomization groups, stratified by sex. Phenotypes A, B and I were characterized as follows: A: sdLDL <40% of LDL, I: sdLDL 40-50% of LDL particles, B: sdLDL >50% of LDL particles. There was no statistically significant difference in the prevalence of phenotype B between groups
Discussion
We found no association between fish oil supplementation during pregnancy and lipid and lipoprotein profile in the 19-year-old offspring. The LDL phenotype in the FO group tended to be healthier in comparison to the phenotype of the OO group, but the difference was not statistically significant. Finally, there were indications of a healthier lipid profile among offspring of mothers with a low baseline fish intake in the FO group, but with only 46 participants in this group, the confidence intervals were wide and no statistically significant association could be demonstrated.
Loss to follow-up was present overall but significantly higher in the FO group compared to the OO group. This could potentially have led to bias, assuming that a lower participation rate was associated with an unhealthy lipid profile. Participation per se would not be expected to be directly associated with the lipid profile, since most of the participants probably were unaware of their lipid levels, but an unhealthy lipid profile is often associated with a high BMI, and participation could be negatively associated with BMI. However, according to the questionnaire data (Table 1), participation was not associated with BMI. Hence, it is unlikely that the results were biased for BMI.
The larger loss to follow-up in the FO group may partly be caused by differences in compliance. Compliance was higher among participants in the FO group compared to the non-participants. By primarily including participants with high compliance in the analyses, this would strengthen a possible association between FO supplementation and later lipid profile. Hence, this cannot explain the finding of no association in the present study. However, those complying with the study protocol might be different from those not complying, and hence this could introduce some residual confounding.
Participation was associated with exposure and may also be associated with e.g. lifestyle factors which could influence lipid profile. Hence, such factors might confound the association between FO supplementation and the lipid profile. In the main analysis we only adjusted for sex. Additional adjustment for offspring smoking and parental overweight did not change estimates.
To our knowledge, no other study has investigated the effect of supplementing with n-3 PUFA during pregnancy on later lipid profile. However, fish oil supplementation during pregnancy has been shown not to affect the lipid profile of the umbilical cord blood speaking against fetal life as the right time window for fish oil supplementation [21]. In another study where term infants were randomized to an intervention aimed at increasing dietary intake of n-3 PUFA and decreasing intake of n-6 PUFA from the time of weaning until the age of 5 years, no association between intervention and lipid profile was found when the children were followed-up at the age of 8 years [18]. The children were, however, very young at follow-up and effects may not be visible until considerably later in life.
Several studies indicate that the lipid profile in adulthood can be influenced, “programmed”, by early nutrition. Hence, Barker et al. showed that a low abdominal circumference at birth was associated with higher concentrations of total cholesterol, LDL cholesterol and Apo-B in adulthood [22]. The authors argued that this could be a consequence of impaired liver growth during late gestation due to malnutrition. Accordingly, a study from the Dutch Hunger Winter showed that persons, who were exposed to hunger during early gestation, displayed significantly higher LDL to HDL ratios, and a tendency towards lower plasma concentrations of HDL and Apo-A1 and higher concentrations of LDL and Apo-B compared to persons born before or after the Dutch Hunger Winter [23]. These results, however, contradict results from the Leningrad Siege, where no association between hunger during fetal life or infancy and any of the lipid parameters in adulthood was found [24].
A large body of research on programming of future lipid profile has focused on the effects of breast feeding. The short-term effect of breast feeding in infants is an increase in total cholesterol [17]. However, this effect may be reversed in adulthood, where breastfeeding has been found to be associated with a lower total cholesterol concentration [17]. Hence, a high total cholesterol concentration early in life may be protective later in life. Also, a study where pre-term infants were randomized to banked breast milk or formulae showed that those receiving banked breast milk had a lower LDL to HDL ratio in adolescence [25]. The mechanism behind the effects of breast-feeding or breast milk on later lipid profile is unknown but may be associated with a lower total energy intake [26] or the higher cholesterol concentration of breast milk compared to infant formulae. The higher cholesterol concentration during early life could potentially suppress endogenous cholesterol production and hence lead to a lower cholesterol concentration later in life [27]. However, breast milk is also high in n-3 PUFA. The short-term effect of supplementing infants with fish oil on serum cholesterol is similar to that of breast feeding [14, 17]. If the effect of breast feeding on later lipid profile is operating through increased cholesterol levels in infancy by inducing decreased endogenous cholesterol production, this could also be the case for fish oil supplementation. Most of the women in the present study had a medium to high baseline fish consumption during pregnancy, and it is possible that this attenuated the effect of fish oil supplementation. Thus, among the offspring of mothers with a low baseline fish intake, there were indications of a beneficial association between fish oil supplementation and later lipid profile. However, as mentioned previously this sub-group analysis contain very few numbers, making it difficult to draw any conclusions. Hence, further studies are needed to confirm this potentially important observation.
Finally, it should be had in mind that very few individuals in early adulthood have an unhealthy lipid profile. Potential benefits of early supplementation with n-3 PUFA on later lipid profile might therefore not be detectable until considerably later in life, and it will be important to follow-up the offspring at later ages.
Conclusion
We found no association between fish oil supplementation during third trimester of pregnancy and offspring plasma lipid and lipoprotein profile in adolescence.
Abbreviations
- Apo-A:
-
Apolipoprotein A1
- Apo-B:
-
Apolipoprotein B100
- CVD:
-
Cardiovascular disease
- PUFA:
-
Polyunsaturated fatty acids
- RCT:
-
Randomized controlled trial
- sdLDL:
-
Small dense LDL particles
- TAG:
-
Triacyglycerols
References
Barker DJ (1997) Fetal nutrition and cardiovascular disease in later life. Br Med Bull 53:96–108
Berenson GS, Srinivasan SR, Bao W, Newman WP III, Tracy RE, Wattigney WA (1998) Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa heart study. N Engl J Med 338:1650–1656
Nguyen QM, Srinivasan SR, Xu JH, Chen W, Berenson GS (2010) Fasting plasma glucose levels within the normoglycemic range in childhood as a predictor of prediabetes and type 2 diabetes in adulthood: the Bogalusa heart study. Arch Pediatr Adolesc Med 164:124–128
Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C (2005) Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ 331:929–934
Adams C, Burke V, Beilin LJ (2005) Cholesterol tracking from childhood to adult mid-life in children from the Busselton study. Acta Paediatr 94:275–280
Elliott WJ (1997) Blood pressure tracking. J Cardiovasc Risk 4:251–256
Harchaoui KE, Visser ME, Kastelein JJ, Stroes ES, Dallinga-Thie GM (2009) Triglycerides and cardiovascular risk. Curr Cardiol Rev 5:216–222
Di AE, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG, Danesh J (2009) Major lipids, apolipoproteins, and risk of vascular disease. JAMA 302:1993–2000
Toft-Petersen AP, Tilsted HH, Aaroe J, Rasmussen K, Christensen T, Griffin BA, Aardestrup IV, Andreasen A, Schmidt EB (2011) Small dense LDL particles—a predictor of coronary artery disease evaluated by invasive and CT-based techniques: a case-control study. Lipids Health Dis 10:21
Gazi IF, Tsimihodimos V, Tselepis AD, Elisaf M, Mikhailidis DP (2007) Clinical importance and therapeutic modulation of small dense low-density lipoprotein particles. Expert Opin Biol Ther 7:53–72
Stampfer MJ, Krauss RM, Ma J, Blanche PJ, Holl LG, Sacks FM, Hennekens CH (1996) A prospective study of triglyceride level, low-density lipoprotein particle diameter, and risk of myocardial infarction. JAMA 276:882–888
Hooper L, Thompson RL, Harrison RA, Summerbell CD, Moore H, Worthington HV, Durrington PN, Ness AR, Capps NE, Davey SG, Riemersma RA, Ebrahim SB (2004) Omega 3 fatty acids for prevention and treatment of cardiovascular disease. Cochrane Database Syst Rev CD003177
Griffin BA (2001) The effect of n-3 fatty acids on low density lipoprotein subfractions. Lipids 36(Suppl):S91–S97
Damsgaard CT, Schack-Nielsen L, Michaelsen KF, Fruekilde MB, Hels O, Lauritzen L (2006) Fish oil affects blood pressure and the plasma lipid profile in healthy Danish infants. J Nutr 136:94–99
Thorsdottir I, Gunnarsdottir I, Palsson GI (2003) Birth weight, growth and feeding in infancy: relation to serum lipid concentration in 12-month-old infants. Eur J Clin Nutr 57:1479–1485
Olsen SF (1994) Further on the association between retarded foetal growth and adult cardiovascular disease. Could low intake or marine diets be a common cause? J Clin Epidemiol 47:565–569
Owen CG, Whincup PH, Odoki K, Gilg JA, Cook DG (2002) Infant feeding and blood cholesterol: a study in adolescents and a systematic review. Pediatrics 110:597–608
Ayer JG, Harmer JA, Xuan W, Toelle B, Webb K, Almqvist C, Marks GB, Celermajer DS (2009) Dietary supplementation with n-3 polyunsaturated fatty acids in early childhood: effects on blood pressure and arterial structure and function at age 8 y. Am J Clin Nutr 90:438–446
Olsen SF, Sorensen JD, Secher NJ, Hedegaard M, Henriksen TB, Hansen HS, Grant A (1992) Randomised controlled trial of effect of fish-oil supplementation on pregnancy duration. Lancet 339:1003–1007
Davies IG, Graham JM, Griffin BA (2003) Rapid separation of LDL subclasses by iodixanol gradient ultracentrifugation. Clin Chem 49:1865–1872
Barden AE, Dunstan JA, Beilin LJ, Prescott SL, Mori TA (2006) n-3 fatty acid supplementation during pregnancy in women with allergic disease: effects on blood pressure, and maternal and fetal lipids. Clin Sci 111:289–294
Barker DJ, Martyn CN, Osmond C, Hales CN, Fall CH (1993) Growth in utero and serum cholesterol concentrations in adult life. BMJ 307:1524–1527
Roseboom TJ, van der Meulen JH, Osmond C, Barker DJ, Ravelli AC, Bleker OP (2000) Plasma lipid profiles in adults after prenatal exposure to the Dutch famine. Am J Clin Nutr 72:1101–1106
Stanner SA, Bulmer K, Andres C, Lantseva OE, Borodina V, Poteen VV, Yudkin JS (1997) Does malnutrition in utero determine diabetes and coronary heart disease in adulthood? Results from the Leningrad siege study, a cross-sectional study. BMJ 315:1342–1348
Singhal A, Cole TJ, Fewtrell M, Lucas A (2004) Breastmilk feeding and lipoprotein profile in adolescents born preterm: follow-up of a prospective randomised study. Lancet 363:1571–1578
Singhal A, Lucas A (2004) Early origins of cardiovascular disease: is there a unifying hypothesis? Lancet 363:1642–1645
Wong WW, Hachey DL, Insull W, Opekun AR, Klein PD (1993) Effect of dietary cholesterol on cholesterol synthesis in breast-fed and formula-fed infants. J Lipid Res 34:1403–1411
Rytter D, Bech BH, Christensen JH, Schmidt EB, Henriksen TB, Olsen SF (2011) Intake of fish oil during pregnancy and adiposity in the 19-y-old offspring: follow-up on a randomized controlled trial. Am J Clin Nutr. doi:10.3945/ajcn.111.014969
Acknowledgments
S.F. Olsen was responsible for the original pregnancy trial and initiated the follow-up of the offspring. D. Rytter, J.H. Christensen, B.H. Bech, S.F. Olsen, E.B. Schmidt and T.B. Henriksen designed the research; D. Rytter and B.H. Bech conducted the research; D. Rytter analyzed the data and wrote the first draft; D. Rytter, J.H. Christensen, S.F. Olsen, E.B. Schmidt, B.H. Bech and T.B. Henriksen wrote the paper; D. Rytter had primary responsibility for the final content. All authors read and approved the final manuscript.
The project described was supported by Award Number R21AT004603 from the National Center For Complementary & Alternative Medicine. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Complementary & Alternative Medicine or the National Institutes of Health. In addition, the follow-up was supported by the EU FP6 consortium, Early Nutrition Programming Project (EARNEST, Project No. FOOD-CT-2005-007036), The Danish Strategic Research Council, The Danish Heart Foundation, The Novo Nordisk Foundation, The Danish Diabetes Foundation and The Aase and Ejnar Danielsens Foundation. The study sponsors were not involved in the study design; in the collection, analysis, or interpretation of data; in the writing of the report, or in the decision to submit the article for publication.
Conflict of interest
All authors declare that there are no conflicts of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Rytter, D., Schmidt, E.B., Bech, B.H. et al. Fish Oil Supplementation During Late Pregnancy Does Not Influence Plasma Lipids or Lipoprotein Levels in Young Adult Offspring. Lipids 46, 1091–1099 (2011). https://doi.org/10.1007/s11745-011-3606-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-011-3606-5