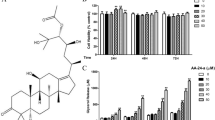
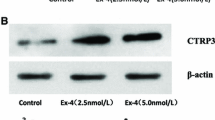
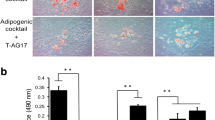
Abstract
Signaling by mTOR complex 1 (mTORC1) promotes anabolic cellular processes in response to growth factors, nutrients, and hormonal cues. Numerous clinical trials employing the mTORC1 inhibitor rapamycin (aka sirolimus) to immuno-suppress patients following organ transplantation have documented the development of hypertriglyceridemia and elevated serum free fatty acids (FFA). We therefore investigated the cellular role of mTORC1 in control of triacylglycerol (TAG) metabolism using cultured murine 3T3-L1 adipocytes. We found that treatment of adipocytes with rapamycin reduced insulin-stimulated TAG storage ~50%. To determine whether rapamycin reduces TAG storage by upregulating lipolytic rate, we treated adipocytes in the absence and presence of rapamycin and isoproterenol, a β2-adrenergic agonist that activates the cAMP/protein kinase A (PKA) pathway to promote lipolysis. We found that rapamycin augmented isoproterenol-induced lipolysis without altering cAMP levels. Rapamycin enhanced the isoproterenol-stimulated phosphorylation of hormone sensitive lipase (HSL) on Ser-563 (a PKA site), but had no effect on the phosphorylation of HSL S565 (an AMPK site). Additionally, rapamycin did not affect the isoproterenol-mediated phosphorylation of perilipin, a protein that coats the lipid droplet to initiate lipolysis upon phosphorylation by PKA. These data demonstrate that inhibition of mTORC1 signaling synergizes with the β-adrenergic-cAMP/PKA pathway to augment phosphorylation of HSL to promote hormone-induced lipolysis. Moreover, they reveal a novel metabolic function for mTORC1; mTORC1 signaling suppresses lipolysis, thus augmenting TAG storage.






Similar content being viewed by others
Abbreviations
- ATGL:
-
Adipocyte triacylglycerol lipase
- AMPK:
-
AMP activated protein kinase
- ATP:
-
Adenosine triphosphate
- cAMP:
-
Cyclic adenosine monophosphate
- eIF4E:
-
Eukaryotic initiation factor-4E
- 4EBP1/PHAS-I:
-
eIF-4E-binding protein or protein-1/heat and acid stable-activated by insulin
- HEAT:
-
Huntington elongation factor 3, the A subunit of protein phosphatase 2A, and TOR1
- HSL:
-
Hormone sensitive lipase
- FBS:
-
Fetal bovine serum
- FRAP:
-
FKBP-12 rapamycin associated protein
- FRB:
-
FKBP12-rapamycin binding domain
- FFA:
-
Free fatty acids
- FKBP12:
-
FK506 binding protein 12
- GAP:
-
GTPase activating protein
- GβL:
-
G protein β subunit-like protein also known as mLST8
- GLUT 4:
-
Glucose transporter 4
- IRS:
-
Insulin receptor substrate
- LKB:
-
Tumor suppressor protein
- LPAAT:
-
Lysophosphatidic acid acyl transferase
- MEFs:
-
Mouse embryonic fibroblasts
- MGL:
-
Monoacylglycerol lipase
- mTOR:
-
Mammalian target of rapamycin (TOR)
- mTOR P-S2481:
-
mTOR phosphorylated on serine 2481
- mTORC1:
-
Mammalian target of rapamycin complex 1
- mTORC2:
-
Mammalian target of rapamycin complex 2
- NCS:
-
Newborn calf serum
- NEFA:
-
Nonesterified fatty acids
- RAPA:
-
Rapamycin
- Raptor:
-
Regulatory associated protein of mammalian target of rapamycin
- Rheb:
-
Ras homolog enriched in brain
- Rictor:
-
Rapamycin-insensitive companion of mTOR
- RII:
-
Regulatory subunit II of PKA
- TOS:
-
TOR signaling motif
- PA:
-
Phosphatidic acid
- PDE:
-
Phosphodiesterase
- PLD:
-
Phospholipase D
- PI3K:
-
Phosphatidylinositol 3-OH kinase
- Pol I:
-
Polymerase I
- PKA:
-
Protein kinase A
- PKB/AKT:
-
Protein kinase B
- PPAR γ:
-
Peroxisome proliferator-activated receptor-γ
- PTEN:
-
Phosphatase and tensin homologue deleted on chromosome 10
- S6K1:
-
p70 ribosomal protein S6 kinase 1
- S6K1 P-T389:
-
S6K1 phosphorylated on threonine 389
- TAG:
-
Triacylglycerol
- TSC:
-
Tuberous sclerosis complex
- VLDL:
-
Very low density lipoprotein
References
Fingar DC, Blenis J (2004) Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene 23:3151–3171
Soliman GA (2005) The mammalian target of rapamycin signaling network and gene regulation. Curr Opin Lipidol 16:317–323
Foster KG, Fingar DC (2010) Mammalian target of rapamycin (mTOR): conducting the cellular signaling symphony. J Biol Chem 285:14071–14077
Jung CH, Ro SH, Cao J, Otto NM, Kim DH (2010) mTOR regulation of autophagy. FEBS Lett 584:1287–1295
Guertin DA, Sabatini DM (2009) The pharmacology of mTOR inhibition. Sci Signal 2:pe24
Oshiro N, Yoshino K, Hidayat S, Tokunaga C, Hara K, Eguchi S, Avruch J, Yonezawa K (2004) Dissociation of raptor from mTOR is a mechanism of rapamycin-induced inhibition of mTOR function. Genes Cells 9:359–366
Soliman GA, Acosta-Jaquez HA, Dunlop EA, Ekim B, Maj NE, Tee AR, Fingar DC (2010) mTOR Ser-2481 autophosphorylation monitors mTORC-specific catalytic activity and clarifies rapamycin mechanism of action. J Biol Chem 285:7866–7879
Kahan BD (2004) Sirolimus: a ten-year perspective. Transplant Proc 36:71–75
Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, Colombo A, Schuler G, Barragan P, Guagliumi G, Molnar F, Falotico R (2002) A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med 346:1773–1780
Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA (2009) Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460:392–395
Morrisett JD, Abdel-Fattah G, Hoogeveen R, Mitchell E, Ballantyne CM, Pownall HJ, Opekun AR, Jaffe JS, Oppermann S, Kahan BD (2002) Effects of sirolimus on plasma lipids, lipoprotein levels, and fatty acid metabolism in renal transplant patients. J Lipid Res 43:1170–1180
Mathe D, Adam R, Malmendier C, Gigou M, Lontie JF, Dubois D, Martin C, Bismuth H, Jacotot B (1992) Prevalence of dyslipidemia in liver transplant recipients. Transplantation 54:167–170
Teutonico A, Schena PF, Di Paolo S (2005) Glucose metabolism in renal transplant recipients: effect of calcineurin inhibitor withdrawal and conversion to sirolimus. J Am Soc Nephrol 16:3128–3135
Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G (2004) Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 431:200–205
Margolin K, Longmate J, Baratta T, Synold T, Christensen S, Weber J, Gajewski T, Quirt I, Doroshow JH (2005) CCI-779 in metastatic melanoma: a phase II trial of the California Cancer Consortium. Cancer 104:1045–1048
Ahima RS (2006) Adipose tissue as an endocrine organ. Obesity (Silver Spring) 14(Suppl 5):242S–249S
Cawthorn WP, Sethi JK (2008) TNF-alpha and adipocyte biology. FEBS Lett 582:117–131
Egan JJ, Greenberg AS, Chang MK, Wek SA, Moos MC Jr, Londos C (1992) Mechanism of hormone-stimulated lipolysis in adipocytes: translocation of hormone-sensitive lipase to the lipid storage droplet. Proc Natl Acad Sci USA 89:8537–8541
Wang S, Soni KG, Semache M, Casavant S, Fortier M, Pan L, Mitchell GA (2008) Lipolysis and the integrated physiology of lipid energy metabolism. Mol Genet Metab 95:117–126
Cohen AW, Razani B, Schubert W, Williams TM, Wang XB, Iyengar P, Brasaemle DL, Scherer PE, Lisanti MP (2004) Role of caveolin-1 in the modulation of lipolysis and lipid droplet formation. Diabetes 53:1261–1270
Su CL, Sztalryd C, Contreras JA, Holm C, Kimmel AR, Londos C (2003) Mutational analysis of the hormone-sensitive lipase translocation reaction in adipocytes. J Biol Chem 278:43615–43619
Saha PK, Kojima H, Martinez-Botas J, Sunehag AL, Chan L (2004) Metabolic adaptations in the absence of perilipin: increased beta-oxidation and decreased hepatic glucose production associated with peripheral insulin resistance but normal glucose tolerance in perilipin-null mice. J Biol Chem 279:35150–35158
Brasaemle DL, Rubin B, Harten IA, Gruia-Gray J, Kimmel AR, Londos C (2000) Perilipin A increases triacylglycerol storage by decreasing the rate of triacylglycerol hydrolysis. J Biol Chem 275:38486–38493
Martinez-Botas J, Anderson JB, Tessier D, Lapillonne A, Chang BH, Quast MJ, Gorenstein D, Chen KH, Chan L (2000) Absence of perilipin results in leanness and reverses obesity in Lepr(db/db) mice. Nat Genet 26:474–479
Aggarwal D, Fernandez ML, Soliman GA (2006) Rapamycin, an mTOR inhibitor, disrupts triglyceride metabolism in guinea pigs. Metabolism 55:794–802
Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM (1998) RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci USA 95:1432–1437
Holm C (2003) Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem Soc Trans 31:1120–1124
Kishida K, Kuriyama H, Funahashi T, Shimomura I, Kihara S, Ouchi N, Nishida M, Nishizawa H, Matsuda M, Takahashi M, Hotta K, Nakamura T, Yamashita S, Tochino Y, Matsuzawa Y (2000) Aquaporin adipose, a putative glycerol channel in adipocytes. J Biol Chem 275:20896–20902
Chen JC, Powers T (2006) Coordinate regulation of multiple and distinct biosynthetic pathways by TOR and PKA kinases in S. cerevisiae. Curr Genet 49:281–293
Manni S, Mauban JH, Ward CW, Bond M (2008) Phosphorylation of the cAMP-dependent protein kinase (PKA) regulatory subunit modulates PKA-AKAP interaction, substrate phosphorylation, and calcium signaling in cardiac cells. J Biol Chem 283:24145–24154
Budillon A, Cereseto A, Kondrashin A, Nesterova M, Merlo G, Clair T, Cho-Chung YS (1995) Point mutation of the autophosphorylation site or in the nuclear location signal causes protein kinase A RII beta regulatory subunit to lose its ability to revert transformed fibroblasts. Proc Natl Acad Sci USA 92:10634–10638
Elliott MR, Shanks RA, Khan IU, Brooks JW, Burkett PJ, Nelson BJ, Kyttaris V, Juang YT, Tsokos GC, Kammer GM (2004) Down-regulation of IL-2 production in T lymphocytes by phosphorylated protein kinase A-RIIbeta. J Immunol 172:7804–7812
Sztalryd C, Xu G, Dorward H, Tansey JT, Contreras JA, Kimmel AR, Londos C (2003) Perilipin A is essential for the translocation of hormone-sensitive lipase during lipolytic activation. J Cell Biol 161:1093–1103
Garton AJ, Campbell DG, Cohen P, Yeaman SJ (1988) Primary structure of the site on bovine hormone-sensitive lipase phosphorylated by cyclic AMP-dependent protein kinase. FEBS Lett 229:68–72
Anthonsen MW, Ronnstrand L, Wernstedt C, Degerman E, Holm C (1998) Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J Biol Chem 273:215–221
Shen WJ, Patel S, Natu V, Kraemer FB (1998) Mutational analysis of structural features of rat hormone-sensitive lipase. Biochemistry 37:8973–8979
Donsmark M, Langfort J, Holm C, Ploug T, Galbo H (2004) Contractions induce phosphorylation of the AMPK site Ser565 in hormone-sensitive lipase in muscle. Biochem Biophys Res Commun 316:867–871
Donsmark M, Langfort J, Holm C, Ploug T, Galbo H (2004) Regulation and role of hormone-sensitive lipase in rat skeletal muscle. Proc Nutr Soc 63:309–314
Zechner R, Kienesberger PC, Haemmerle G, Zimmermann R, Lass A (2009) Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J Lipid Res 50:3–21
Wei E, Gao W, Lehner R (2007) Attenuation of adipocyte triacylglycerol hydrolase activity decreases basal fatty acid efflux. J Biol Chem 282:8027–8035
Zurita-Martinez SA, Cardenas ME (2005) Tor and cyclic AMP-protein kinase A: two parallel pathways regulating expression of genes required for cell growth. Eukaryot Cell 4:63–71
Soulard A, Cremonesi A, Moes S, Schutz F, Jeno P, Hall MN (2010) The rapamycin-sensitive phosphoproteome reveals that TOR controls protein kinase A toward some but not all substrates. Mol Biol Cell 21:3475–3486
Chakrabarti P, English T, Shi J, Smas CM, Kandror KV (2010) Mammalian target of rapamycin complex 1 suppresses lipolysis, stimulates lipogenesis, and promotes fat storage. Diabetes 59:775–781
Laplante M, Sabatini DM (2009) mTOR signaling at a glance. J Cell Sci 122:3589–3594
Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, Griffiths JR, Chung YL, Schulze A (2008) SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab 8:224–236
Kim JE, Chen J (2004) regulation of peroxisome proliferator-activated receptor-gamma activity by mammalian target of rapamycin and amino acids in adipogenesis. Diabetes 53:2748–2756
Laplante M, Sabatini DM (2010) mTORC1 activates SREBP-1c and uncouples lipogenesis from gluconeogenesis. Proc Natl Acad Sci USA 107:3281–3282
Leibowitz G, Cerasi E, Ketzinel-Gilad M (2008) The role of mTOR in the adaptation and failure of beta-cells in type 2 diabetes. Diabetes Obes Metab 10(Suppl 4):157–169
Teleman AA, Chen YW, Cohen SM (2005) Drosophila Melted modulates FOXO and TOR activity. Dev Cell 9:271–281
Pende M, Kozma SC, Jaquet M, Oorschot V, Burcelin R, Le Marchand-Brustel Y, Klumperman J, Thorens B, Thomas G (2000) Hypoinsulinaemia, glucose intolerance and diminished beta-cell size in S6K1-deficient mice. Nature 408:994–997
Le Bacquer O, Petroulakis E, Paglialunga S, Poulin F, Richard D, Cianflone K, Sonenberg N (2007) Elevated sensitivity to diet-induced obesity and insulin resistance in mice lacking 4E-BP1 and 4E-BP2. J Clin Invest 117:387–396
Acknowledgments
The authors would like to express their gratitude to Drs. Nancy Weigel (Baylor College of Medicine) and Victoria Knutson (University of Texas) for sharing reagents, encouragement, support, and advice. Funding: This work was funded by grants from the National Institutes of Health (K01 DK60654) and the American Heart Association (0750060Z) to GS and NIH-R01 (DK-078135) to DCF.
Conflict of interest
Nothing to disclose; there are no commercial or other associations that may pose a conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Soliman, G.A., Acosta-Jaquez, H.A. & Fingar, D.C. mTORC1 Inhibition via Rapamycin Promotes Triacylglycerol Lipolysis and Release of Free Fatty Acids in 3T3-L1 Adipocytes. Lipids 45, 1089–1100 (2010). https://doi.org/10.1007/s11745-010-3488-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-010-3488-y