Abstract
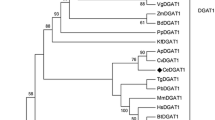
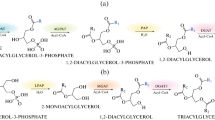
Boraginaceae species, such as those from the genus Echium, contain high levels of the Δ6-desaturated γ-linolenic (18:3n-6) and octadecatetraenoic (18:4n-3) acids. These are unusual fatty acids among the plant kingdom that are gaining interest due to their benefits to human health. The potential utility of acyltransferases aimed at an increase in oil yield and fatty acid profiling has been reported. In this work, a gene encoding an acyl-CoA:diacylglycerol acyltransferase (DGAT, EC 2.3.1.20) was cloned from Echium pitardii. Genomic and cDNA sequences obtained revealed a gene structure composed of 16 exons, yielding a protein (EpDGAT) of 473 amino acids with high similarity to DGAT1 enzymes of plants. Protein features such as a predicted structure with a highly hydrophilic N-terminus followed by 10 transmembrane domains, as well as the presence of diverse specific signatures, also indicate that EpDGAT belongs to the DGAT1 family. indeed. DGAT activity of the protein encoded by EpDGAT was confirmed by heterologous expression of the full-length cDNA in a yeast mutant (H1246) defective in the synthesis of triacylglycerols. Fatty acid composition of the triacylglycerols synthesized by EpDGAT in H1246 yeast cultures supplemented with polyunsaturated fatty acids suggest a substrate preference for the trienoic fatty acids α-linolenic acid (18:3n-3) and γ-linolenic acid over the dienoic linoleic acid (18:2n-6). Site-directed mutagenesis has revealed the presence of a critical residue (P178 in EpDGAT) within a reported thiolase signature for binding of acyl-enzyme intermediates that might be involved in the active site of the enzyme. Transcript analysis for EpDGAT shows an ubiquitous expression of the gene which is increased in leaves during senescence.







Similar content being viewed by others
Abbreviations
- ALA:
-
Alpha-linolenic acid
- cDNA:
-
Complementary DNA
- CTAB:
-
Cetyl trimethylammonium bromide
- DAG:
-
Diacylglycerol
- DGAT:
-
Acyl-CoA:diacylglycerol acyltransferase
- DIG:
-
Digoxigenin
- FFA:
-
Free fatty acids
- GC:
-
Gas chromatography
- GLA:
-
Gamma-linolenic acid
- IPCR:
-
Inverse PCR
- LNA:
-
Linoleic acid
- NL:
-
Neutral lipids
- PCR:
-
Polymerase chain reaction
- PL:
-
Polar lipids
- PUFA:
-
Polyunsaturated fatty acid
- RT-PCR:
-
Reverse transcriptase PCR
- SDS:
-
Sodium dodecylsulfate
- SE:
-
Steryl esters
- ST:
-
Sterols
- TAG:
-
Triacylglycerol
- TL:
-
Total lipids
- TLC:
-
Thin layer chromatography
References
Dyer JM, Stymne S, Green AG, Carlsson AS (2008) High-value oils from plants. Plant J 54:640–655
Durrett TP, Benning C, Ohlrogge J (2008) Plant triacylglycerols as feedstocks for the production of biofuels. Plant J 54:593–607
Zheng P, Allen WB, Roesler K, Williams ME et al (2008) A phenylalanine in DGAT is a key determinant of oil content and composition in maize. Nat Genet 40:367–372
Dircks L, Sul HS (1999) Acyltransferases of de novo glycerophospholipid biosynthesis. Prog Lipid Res 38:461–479
Lehner R, Kuksis A (1996) Biosynthesis of triacylglycerols. Prog Lipid Res 35:169–201
Coleman RA, Lee DP (2004) Enzymes of triacylglycerol synthesis and their regulation. Prog Lipid Res 43:134–176
Xu J, Francis T, Mietkiewska E, Giblin EM et al (2008) Cloning and characterization of an acyl-CoA-dependent diacylglycerol acyltransferase 1 (DGAT1) gene from Tropaeolum majus, and a study of the functional motifs of the DGAT protein using site-directed mutagenesis to modify enzyme activity and oil content. Plant Biotechnol J 6:799–818
Stobart AK, Mancha M, Lenman M, Dahlqvist A, Stymne S (1997) Triacylglycerols are synthesized and utilized by transacylation reactions in microsomal preparations of developing safflower (Carthamus tinctorius L.) seeds. Planta 203:58–66
Dahlqvist A, Stahl U, Lenman M, Banas A et al (2000) Phospholipid:diacylglycerol acyltransferase: an enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants. Proc Natl Acad Sci USA 97:6487–6492
Millar AA, Smith MA, Kunst L (2000) All fatty acids are not equal: discrimination in plant membrane lipids. Trends Plant Sci 5:95–101
Banas A, Carlsson AS, Huang B, Lenman M et al (2005) Cellular sterol ester synthesis in plants is performed by an enzyme (phospholipid:sterol acyltransferase) different from the yeast and mammalian acyl-CoA:sterol acyltransferases. J Biol Chem 280:34626–34634
Mhaske V, Beldjilali K, Ohlrogge J, Pollard M (2005) Isolation and characterization of an Arabidopsis thaliana knockout line for phospholipid: diacylglycerol transacylase gene (At5g13640). Plant Physiol Biochem 43:413–417
Kroon JT, Wei W, Simon WJ, Slabas AR (2006) Identification and functional expression of a type 2 acyl-CoA:diacylglycerol acyltransferase (DGAT2) in developing castor bean seeds which has high homology to the major triglyceride biosynthetic enzyme of fungi and animals. Phytochemistry 67:2541–2549
Cahoon EB, Shockey JM, Dietrich CR, Gidda SK et al (2007) Engineering oilseeds for sustainable production of industrial and nutritional feedstocks: solving bottlenecks in fatty acid flux. Curr Opin Plant Biol 10:236–244
Mancha M, Osorio J, Garces R, Ruso J et al (1994) New sunflower mutants with altered seed fatty acid composition. Prog Lipid Res 33:147–154
Bao X, Ohlrogge J (1999) Supply of fatty acid is one limiting factor in the accumulation of triacylglycerol in developing embryos. Plant Physiol 120:1057–1062
Katavic V, Reed DW, Taylor DC, Giblin EM et al (1995) Alteration of seed fatty acid composition by an ethyl methane sulfonate-induced mutation in Arabidopsis thaliana affecting diacylglycerol acyltransferase activity. Plant Physiol 108:399–409
Jako C, Kumar A, Wei Y, Zou J et al (2001) Seed-specific over-expression of an Arabidopsis cDNA encoding a diacylglycerol acyltransferase enhances seed oil content and seed weight. Plant Physiol 126:861–874
Settlage SB, Kwanyuen P, Wilson RF (1998) Relation between diacylglycerol acyltransferase activity and oil concentration in soybean. J Am Oil Chem Soc 75:775–781
Perry HJ, Bligny R, Gout E, Harwood JL (1999) Changes in Kennedy pathway intermediates associated with increased triacylglycerol synthesis in oil-seed rape. Phytochemistry 52:799–804
Giannoulia K, Haralampidis K, Poghosyan Z, Murphy DJ, Hatzopoulos P (2000) Differential expression of diacylglycerol acyltransferase (DGAT) genes in olive tissues. Biochem Soc Trans 28:695–697
Triki S, Ben Hamida J, Mazliak P (2000) Diacylglycerol acyltransferase in maturing sunflower seeds. Biochem Soc Trans 28:689–692
Lung SC, Weselake RJ (2006) Diacylglycerol acyltransferase: a key mediator of plant triacylglycerol synthesis. Lipids 41:1073–1088
Frentzen M (1998) Acyltransferases from basic science to modified seed oils. Fett-Lipid 100:161–166
Thelen JJ, Ohlrogge JB (2002) Metabolic engineering of fatty acid biosynthesis in plants. Metab Eng 4:12–21
Dyer JM, Mullen RT (2005) Development and potential of genetically engineered oilseeds. Seed Sci Res 15:255–267
Lardizabal KD, Effertz R, Levering CK, Mai JT et al (2008) Expression of Umbelopsis ramanniana DGAT2A in seed increases oil in soybean. Plant Physiol 148:89–96
Ichihara K, Takahashi T, Fujii S (1988) Diacylglycerol acyltransferase in maturing safflower seeds: its influences on the fatty acid composition of triacylglycerol and on the rate of triacylglycerol synthesis. Biochim Biophys Acta 958:125–129
Vogel G, Browse J (1996) Cholinephosphotransferase and diacylglycerol acyltransferase (substrate specificities at a key branch point in seed lipid metabolism). Plant Physiol 110:923–931
Daniel J, Abraham L, Balaji K, Rajasekharan R (2003) Biosynthesis of stearate-rich triacylglycerol in developing embryos and microsomal membranes from immature seeds of Garcinia indica Chois. Curr Sci 85:363–370
Yu K, McCracken CT Jr, Li R, Hildebrand DF (2006) Diacylglycerol acyltransferases from Vernonia and Stokesia prefer substrates with vernolic acid. Lipids 41:557–566
Cases S, Smith SJ, Zheng YW, Myers HM et al (1998) Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc Natl Acad Sci USA 95:13018–13023
Hobbs DH, Lu C, Hills MJ (1999) Cloning of a cDNA encoding diacylglycerol acyltransferase from Arabidopsis thaliana and its functional expression. FEBS Lett 452:145–149
Routaboul JM, Benning C, Bechtold N, Caboche M, Lepiniec L (1999) The TAG1 locus of Arabidopsis encodes for a diacylglycerol acyltransferase. Plant Physiol Biochem 37:831–840
Zou J, Wei Y, Jako C, Kumar A et al (1999) The Arabidopsis thaliana TAG1 mutant has a mutation in a diacylglycerol acyltransferase gene. Plant J 19:645–653
Bouvier-Nave P, Benveniste P, Oelkers P, Sturley SL, Schaller H (2000) Expression in yeast and tobacco of plant cDNAs encoding acyl CoA:diacylglycerol acyltransferase. Eur J Biochem 267:85–96
Nykiforuk CL, Furukawa-Stoffer TL, Huff PW, Sarna M et al (2002) Characterization of cDNAs encoding diacylglycerol acyltransferase from cultures of Brassica napus and sucrose-mediated induction of enzyme biosynthesis. Biochim Biophys Acta 1580:95–109
He X, Turner C, Chen GQ, Lin JT, McKeon TA (2004) Cloning and characterization of a cDNA encoding diacylglycerol acyltransferase from castor bean. Lipids 39:311–318
Milcamps A, Tumaney AW, Paddock T, Pan DA et al (2005) Isolation of a gene encoding a 1, 2-diacylglycerol-sn-acetyl-CoA acetyltransferase from developing seeds of Euonymus alatus. J Biol Chem 280:5370–5377
Wang HW, Zhang JS, Gai JY, Chen SY (2006) Cloning and comparative analysis of the gene encoding diacylglycerol acyltransferase from wild type and cultivated soybean. Theor Appl Genet 112:1086–1097
Shockey JM, Gidda SK, Chapital DC, Kuan JC et al (2006) Tung tree DGAT1 and DGAT2 have nonredundant functions in triacylglycerol biosynthesis and are localized to different subdomains of the endoplasmic reticulum. Plant Cell 18:2294–2313
Lardizabal KD, Mai JT, Wagner NW, Wyrick A et al (2001) DGAT2 is a new diacylglycerol acyltransferase gene family: purification, cloning, and expression in insect cells of two polypeptides from Mortierella ramanniana with diacylglycerol acyltransferase activity. J Biol Chem 276:38862–38869
Turkish AR, Henneberry AL, Cromley D, Padamsee M et al (2005) Identification of two novel human acyl-CoA wax alcohol acyltransferases: members of the diacylglycerol acyltransferase 2 (DGAT2) gene superfamily. J Biol Chem 280:14755–14764
Saha S, Enugutti B, Rajakumari S, Rajasekharan R (2006) Cytosolic triacylglycerol biosynthetic pathway in oilseeds. Molecular cloning and expression of peanut cytosolic diacylglycerol acyltransferase. Plant Physiol 141:1533–1543
Lu CL, de Noyer SB, Hobbs DH, Kang J et al (2003) Expression pattern of diacylglycerol acyltransferase-1, an enzyme involved in triacylglycerol biosynthesis, in Arabidopsis thaliana. Plant Mol Biol 52:31–41
Zhang FY, Yang MF, Xu YN (2005) Silencing of DGAT1 in tobacco causes a reduction in seed oil content. Plant Sci 169:689–694
Burgal J, Shockey J, Lu C, Dyer J et al (2008) Metabolic engineering of hydroxy fatty acid production in plants: RcDGAT2 drives dramatic increases in ricinoleate levels in seed oil. Plant Biotechnol J 6:819–831
Chen GQ, Turner C, He X, Nguyen T et al (2007) Expression profiles of genes involved in fatty acid and triacylglycerol synthesis in castor bean (Ricinus communis L.). Lipids 42:263–274
Guil-Guerrero JL, Gomez-Mercado F, Rodriguez-Garcia I, Campra-Madrid P, Garcia-Maroto F (2001) Occurrence and characterization of oils rich in gamma-linolenic acid (III): the taxonomical value of the fatty acids in Echium (Boraginaceae). Phytochemistry 58:117–120
Garcia-Maroto F, Garrido-Cardenas JA, Rodriguez-Ruiz J, Vilches-Ferron M et al (2002) Cloning and molecular characterization of the Delta 6-desaturase from two Echium plant species: production of GLA by heterologous expression in yeast and tobacco. Lipids 37:417–426
Sandager L, Gustavsson MH, Stahl U, Dahlqvist A et al (2002) Storage lipid synthesis is non-essential in yeast. J Biol Chem 277:6478–6482
Ochman H, Ajioka JW, Garza D, Hartl DL (1990) Inverse polymerase chain reaction. Biotechnology 8:759–760
Garcia-Maroto F, Garrido-Cardenas JA, Michaelson LV, Napier JA, Alonso DL (2007) Cloning and molecular characterisation of a Delta(8)-sphingolipid-desaturase from Nicotiana tabacum closely related to Delta(6)-acyl-desaturases. Plant Mol Biol 64:241–250
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Rzhetsky A, Nei M (1992) Statistical properties of the ordinary least-squares, generalized least-squares, and minimum-evolution methods of phylogenetic inference. J Mol Evol 35:367–375
Kumar S, Tamura K, Jakobsen IB, Nei M (2001) MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1244–1245
Taylor B, Powel A (1982) Isolation of plant DNA and RNA. Focus 4:4–6
Elble R (1992) A simple and efficient procedure for transformation of yeasts. BioTechniques 13:18–20
Rodriguez-Ruiz J, Belarbi EH, Sanchez JLG, Alonso DL (1998) Rapid simultaneous lipid extraction and transesterification for fatty acid analyses. Biotechnol Tech 12:689–691
Alonso DL, Belarbi EL, Rodriguez-Ruiz J, Segura CI, Gimenez A (1998) Acyl lipids of three microalgae. Phytochemistry 47:1473–1481
Kates M (1988) Techniques of lipidology: isolation, analysis, and identification of lipids. Elsevier, Amsterdam
Hofmann K, Stoffel W (1993) TMbase—a database of membrane spanning proteins segments. Biol Chem Hoppe-Seyler 374:166
Kaup MT, Froese CD, Thompson JE (2002) A role for diacylglycerol acyltransferase during leaf senescence. Plant Physiol 129:1616–1626
Oh SA, Park JH, Lee GI, Paek KH et al (1997) Identification of three genetic loci controlling leaf senescence in Arabidopsis thaliana. Plant J 12:527–535
John I, Drake R, Farrell A, Cooper W et al (1995) Delayed leaf senescence in ethylene-deficient ACC-oxidase antisense tomato plants: molecular and physiological analysis. Plant J 7:483–490
Athenstaedt K, Daum G (1999) Phosphatidic acid, a key intermediate in lipid metabolism. Eur J Biochem 266:1–16
Griffiths G, Harwood JL (1991) The regulation of triacylglycerol biosynthesis in cocoa (Theobroma cacao) L. Planta 184:279–284
Cao YZ, Huang AH (1987) Acyl coenzyme A preference of diacylglycerol acyltransferase from the maturing seeds of Cuphea, maize, rapeseed, and canola. Plant Physiol 84:762–765
Yu K, Li R, Hatanaka T, Hildebrand D (2008) Cloning and functional analysis of two type 1 diacylglycerol acyltransferases from Vernonia galamensis. Phytochemistry 69:1119–1127
Griffiths G, Stobart AK, Stymne S (1988) Delta 6- and delta 12-desaturase activities and phosphatidic acid formation in microsomal preparations from the developing cotyledons of common borage (Borago officinalis). Biochem J 252:641–647
Cao G, Goldstein JL, Brown MS (1996) Complementation of mutation in acyl-CoA:cholesterol acyltransferase (ACAT) fails to restore sterol regulation in ACAT-defective sterol-resistant hamster cells. J Biol Chem 271:14642–14648
Lewin TM, Wang P, Coleman RA (1999) Analysis of amino acid motifs diagnostic for the sn-glycerol-3-phosphate acyltransferase reaction. Biochemistry 38:5764–5771
Hirst JD, Vieth M, Skolnick J, Brooks CL 3rd (1996) Predicting leucine zipper structures from sequence. Protein Eng 9:657–662
Bornberg-Bauer E, Rivals E, Vingron M (1998) Computational approaches to identify leucine zippers. Nucleic Acids Res 26:2740–2746
Acknowledgments
This work was supported by grants from the Ministerio de Ciencia y Tecnología (MCYT, AGL2005-01498/AGR) and Junta de Andalucía (P05-189-AGR). J.A. Garrido-Cárdenas and A. Mañas-Fernández were recipients of postgraduate fellowships from the MCYT and Junta de Andalucía, respectively. We are also grateful to J. Pérez-Parra and J.C. Gázquez for providing greenhouse facilities and technical assistance in plant culture at the “Estación Experimental Las Palmerillas (CAJAMAR)”. Dr. Stymne (Swedish University of Agricultural Sciences, Uppsala) kindly provided the mutant strain of S. cerevisiae H1246, and is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
11745_2009_3303_MOESM1_ESM.doc
Deduced donor and acceptor splice sites of the EpDGAT gene. Splicing sites were deduced from the comparison between genomic and cDNA sequences. Non-standard acceptor sequence on intron 3 is underlined. (DOC 27 kb)
About this article
Cite this article
Mañas-Fernández, A., Vilches-Ferrón, M., Garrido-Cárdenas, J.A. et al. Cloning and Molecular Characterization of the Acyl-CoA:Diacylglycerol Acyltransferase 1 (DGAT1) Gene from Echium . Lipids 44, 555–568 (2009). https://doi.org/10.1007/s11745-009-3303-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-009-3303-9