Abstract
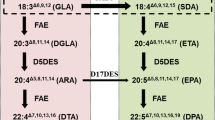
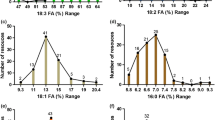
Modern diets are often deficient in ω-3 fatty acids and additional dietary sources of ω-3 fatty acids are useful. In order to investigate the molecular basis of the high accumulation of the ω-3 fatty acid, α-linolenic acid (18:3), in three different plants, flax (Linum usitatissimum), Dracocephalum moldavica, and Perilla frutescens ω-3 desaturase activity, transcript levels, and 18:3 in-vivo synthesis were examined. The 18:3 content was found to be higher at the later developmental stage of D. moldavica (68%) compared with P. frutescens (59%) and flax (45%) cotyledons. The 18:3 and 18:2 contents in both PC and TAG were determined during various stages of seed development for all three plants in addition to soybean (Glycine max). Northern blot analysis data of three different stages of D. moldavica, flax, and P. frutescens compared with moderately low 18:3 producers, soybean (Glycine max), and Arabidopsis thaliana and Brassica napus, (8–10% 18:3) at a stage of zygotic embryo development of high triglyceride synthesis showed that ω-3 desaturase mRNA levels were higher in all three high 18:3 producers, flax, D. moldavica and P. frutescens. This indicates that the high level of α-linolenic acid in TAG may be largely controlled by the level of ω-3 desaturase gene expression. However, the PC versus TAG fatty acid composition data suggested that along with ω-3 desaturase other enzymes also play a role in 18:3 accumulation in TAG, and the high accumulators have a selective transfer of α-linolenic acid into TAG.





Similar content being viewed by others
Abbreviations
- ACP:
-
acyl carrier protein
- ALA:
-
α-linolenic acid
- DAG:
-
diacylglycerol
- DGAT:
-
diacylglycerol acyl transferase
- DHA:
-
docosohexaenoic acid
- EFA, EPA:
-
eicosapentaenoic acid
- ER:
-
endoplasmic reticulum
- FFAP, PC:
-
phosphatidylcholine
- PUFA:
-
polyunsaturated fatty acid
- TAG:
-
triacylglycerol
References
de Lorgeril M, Salen P (2003) Dietary Prevention of coronary heart disease: Focus on omega-6/omega-3 essential fatty acid balance. In: Simpolus A, Cleland L (eds) Omega-6/omega-3 essential fatty acid ratio: the scientific evidence. Basel, Karger, pp 37–56
Trautwein EA (2001) n-3 Fatty acids—physiological and technical aspects for their use. Eur J Lipid Sci Technol 103:45–55
Sprecher H (2002) The roles of anabolic and catabolic reactions in the synthesis and recycling of polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids 67:79–83
de Lorgeril M, Salen P (2004) Alpha-linolenic acid and coronary heart disease. Nutr Metab Cardiovasc Dis 14(3):162–169
Lewis NM, Seburg S, Flanagan NL (2000) Enriched eggs as a source of N-3 polyunsaturated fatty acids for humans. Poult Sci 79(7):971–974
Kuznetsova NV (1978) Variability of fatty acid contents in seed oil of fiber flax cultivars of different origin. Byulleten Vsesoyuznogo Ordana 59:30–36
Yarosh NP, Megorskaya OM, Ivanova ON (1980) Dynamics of fatty acids in the free and bound lipids in ripening seeds of linseed and flax. Trudy po Prikladnoi Botanike, Genetike i Selektsii 66(3):28–36
Stymne S, Tonnet ML, Green AG (1992) Biosynthesis of linolenate in developing embryos and cell-free preparations of high-linolenate linseed (Linum usitatissimum) and low-linolenate mutants. Arch Biochem Biophys 294(2):557–563
Hagemann JM, Earle FR, Wolff IA (1967) Search for new industrial oils. XIV. Seed oils of labiatae. Lipids 2(5):371–380
Hwang SZ, Ko YS (1982) Studies on the constituents of Korean edible oils and fats, 5: analysis of fatty acids in sesame and perilla oil by high performance liquid chromatography. Korean J Nutr 15(1):15–21
Lee JI et al (1986) Study on the evaluation of oil quality and the differences of fatty acids composition between varieties in perilla (Perilla frutescens Britton var. japonica Hara). Korean J Breed (Korean R.) 18(3):228–233
Domokos J, Peredi J, Halasz-Zelnik K (1994) Characterization of seed oils of dragonhead (Dracocephalum moldavica L.) and catnip (Nepeta cataria var. citriodora Balb). Ind Crops Prod 3(1–2):91–94
Matthaus B (1997) Antinutritive compounds in different oilseeds. Fett-Lipid 99(5):170–174
Toyoaki A, Tomoko Yamada, Takehito Kinoshita, Shaikh M. Rahman, Yutaka Takagi (2005) Identification of corresponding genes for three low-α-linolenic acid mutants and elucidation of their contribution to fatty acid biosynthesis in soybean seed. Plant Sci 168:1615–1623
Norby SW, Adams CA, Rinne RW (1984) An ultrastructural study of soybean seed development. in American Soy Association Monograph. University of Ilinois Press, Champagne Urbana, IL
Dahmer ML et al (1989) A rapid screening technique for determining the lipid composition of soybean seeds. J Am Oil Chem Soc 66(4):543–548
Miquel M, Browse JA (1992) Arabidopsis mutants deficient in polyunsaturated fatty acid synthesis. Biochemical and genetic characterization of a plant oleoyl-phosphatidylcholine desaturase. J Biol Chem 267(3):1502–1509
Khan MU, Williams JP (1977) Improved thin-layer chromatographic method for the separation of major phospholipids and glycerolipids from plant lipid extracts and phosphatidylglycerol and bis (monoacylglyceryl) phosphate from animal lipid extracts. J Chromatogr 140:179–185
Lightner J, Wu J, Browse J (1994) A mutant of Arabidopsis with increased levels of stearic acid. Plant Physiol 106:1443–1451
Taylor DC, Weber N, Hogge LR, Underhill EW (1990) A simple enzymatic method for the preparation of radiolabeled erucoyl-CoA and other long-chain fatty acyl-CoAs and their characterization by mass spectrometry. Anal Biochem 184(2):311–316
Stymne S, Lorraine Tonnet M, Green AG (1992) Biosynthesis of linolenate in developing embryos and cell-free preparations of high-linolenate linseed (Linum usitatissimum) and low-linolenate mutants. Arch Biochem Biophys 294(2):557–563
Karcher SJ (1995) Molecular biology: a project approach. Academic Press, Inc., San Diego, CA
Longvah T, Deosthale YG (1991) Chemical a nutritional studies on Hanshi (Perilla frutescens) a traditional oil seed from northeast India. J Am Oil Chem Soc 68:781–784
Longvah T, Deosthale YG, Kumar PU (2000) Nutritional and short term toxicological evaluation of perilla seed oil. Food Chem 70(1):13–16
Privett OS et al (1973) Studies on the lipid composition of developing soybean seeds. J Am Oil Chem Soc 50:516–520
Suh MC, Schultz DJ, Ohlrogge TB (2002) What limits production of unusual monoenoic fatty acids in transgenic plants? Planta 215:584–595
Wilson RF (1987) Soybean: improvement, production and uses. In: Wilcox JR (ed) Seed metabolism. American Society of Agronomy, Madison, WI, pp 643–686
Ohlrogge JB, Browse J (1995) Lipid biosynthesis. Plant Cell 7:957–970
Topfer R, Martini N, Schell J (1995) Modification of plant lipid synthesis. Science 268:681–686
Thomaeus S, Carlsson AS, Stymne S (2001) Distribution of fatty acids in polar and neutral lipids during seed development in Arabidopsis thaliana genetically engineered to produce acetylenic, epoxy and hydroxyl fatty acids. Plant Sci 161(5):997–1003
Horiguchi G, Kodama H, Iba K (2001) Characterization of Arabidopsis mutants that are associated with altered C18 unsaturated fatty acid metabolism. Plant Sci 161:1117–1123
O’Hara P, Slabas AR, Fawectt T (2002) Fatty acid and lipid biosynthetic genes are expressed at constant molar ratios but different absolute levels during embryogenesis. Plant Physiol 129:310–320
Sorensen B, Furukawa-Stoffer TL, Marshall KS, Page EK, Mir Z, Forster RJ, Weselake RJ (2005) Storage lipid accumulation and acyltransferase action in developing flax seed. Lipids 40:1043–1049
Byrum JR et al (1997) Alteration of the ω-3 fatty acid desaturase gene is associated with reduced linolenic acid in the A5 soybean genotype. Theor Appl Genet 94(3–4):356–359
Acknowledgments
This work was supported by a graduate fellowship from Ain Shams University in Cairo Egypt to Mohammed Abdel-Reheem, the United Soybean Board and the KY Agricultural Experiment Station.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Rao, S., Abdel-Reheem, M., Bhella, R. et al. Characteristics of High α-Linolenic Acid Accumulation in Seed Oils. Lipids 43, 749–755 (2008). https://doi.org/10.1007/s11745-008-3207-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-008-3207-0