Abstract
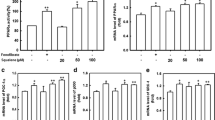
FA with varying chain lengths and an α-methyl group and/or a sulfur in the β-position were tested as peroxisome proliferator-activated receptor (PPAR)α,-δ(β), and-γ ligands by transient transfection in COS-1 cells using chimeric receptor expression plasmids, containing cDNAs encoding the ligand-binding domain of PPARα,-δ, and-γ. For PPARα, an increasing activation was found with increasing chain length of the sulfur-substituted FA up to C14-S acetic acid (tetradecylthioacetic acid=TTA). The derivatives were poor, and nonsignificant, activators of PPARδ. For PPARγ, activation increased with increasing chain length up to C16-S acetic acid. A methyl group was introduced in the α-position of palmitic acid, TTA, EPA, DHA, cis9,trans11CLA, and trans10,cis12 CLA. An increased activation of PPARα was obtained for the α-methyl derivatives compared with the unmethylated FA. This increase also resulted in increased expression of the two PPARα target genes acyl-CoA oxidase and liver FA-binding protein for α-methyl TTA, α-methyl EPA, and α-methyl DHA. Decreased or altered metabolism of these derivatives in the cells cannot be excluded. In conclusion, saturated FA with sulfur in the β-position and increasing carbon chain length from C9−S acetic acid to C14−S acetic acid have increasing effects as activators of PPARα and-γ in transfection assays. Furthermore, α-methyl FA derivatives of a saturated natural FA (palmitic acid), a sulfur-substituted FA (TTA), and PUFA (EPA, DHA, c9,t11 CLA, and t10,c12 CLA) are stronger PPARα activators than the unmethylated compounds.
Similar content being viewed by others
Abbreviations
- AcA:
-
acetic acid
- ACO:
-
acyl-CoA oxidase 1
- BRL 49653:
-
rosiglitazone
- HRMS:
-
high-resolution MS
- LBD:
-
ligand-binding domain
- L-FABP:
-
liver-specific fatty acid-binding protein
- PPAR:
-
peroxisome proliferator-activated receptor
- RXR:
-
retinoid X receptor
- TTA:
-
tetradecylthioacetic acid (C14−S acetic acid)
- USAS:
-
upstream activating sequence
- WY-14,643:
-
pirinixic acid, also know as 4-chloro-6-(2,3-xylidino)-2-pyrimidinylthioacetic acid
References
Brun, R.P., and Spiegelman, B.M. (1997) PPARγ and the Molecular Control of Adipogenesis, J. Endocrinol. 155, 217–218.
Schoonjans, K., Staels, B., and Auwerx, J. (1996) Role of the Peroxisome Proliferator-Activated Receptor (PPAR) in Mediating the Effects of Fibrates and Fatty Acids on Gene Expression, J. Lipid Res. 37, 907–925.
Schoonjans, K., Martin, G., Staels, B., and Auwerx, J. (1997) Peroxisome Proliferator-Activated Receptors, Orphans with Ligands and Functions, Curr. Opin. Lipidol. 8, 159–166.
Sørensen, H.N., Treuter, E., and Gustafsson, J.Å. (1997) Regulation of Peroxisome Proliferiator-Activated Receptors, Vitam. Horm. 54, 121–166.
Isseman, I., and Green, S. (1990) Activation of a Member of the Steroid Hormone Receptor Superfamily by Peroxisome Proliferators, Nature 347, 645–650.
Dreyer, C., Krey, G., Keller, H., Givel, F., Helftenbein, G., and Wahli, W. (1992) Control of the Peroxisomal β-Oxidation Pathway by a Novel Family of Nuclear Hormone Receptors, Cell 68, 879–887.
Kliewer, S.A., Forman, B.M., Blumberg, B., Ong, E.S., Borgmeyer, U., Mangelsdorf, D.J., Umesono, K., and Evans, R.M. (1994) Differential Expression and Activation of a Murine Peroxisomal Proliferator-Activated Receptor, Proc. Natl. Acad. Sci. USA 91, 7355–7359.
Braissant, O., Foufelle, F., Scotto, C., Dauca, M., and Wahli, W. (1996) Differential Expression of Peroxisome-Proliferator Activated Receptors (PPARs): Tissue Distribution of PPAR-α,-β, and-γ in the Adult Rat, Endocrinology 137, 354–366.
Keller, H., Dreyer, C., Medin, J., Mahoudi, A., Ozato, K., and Wahli, W. (1993) Fatty Acids and Retinoids Control Lipid Metabolism Through Activation of Peroxisome Proliferator-Activated Receptor-Retinoid X Receptor Heterodimers, Proc. Natl. Acad. Sci. USA 90, 2160–2164.
Kliewer, S.A., Umesono, K., Noonan, D.J., Heyman, R.A., and Evans, R.M. (1992) Convergence of 9-cis Retinoic Acid and Peroxisome Proliferator Signalling Pathways Through Heterodimer Formation of Their Receptors, Nature 358, 771–774.
Forman, B., Chen, J., and Evans, R.M. (1997) Hypolipidemic Drugs, Polyunsaturated Fatty Acids, and Eicosanoids Are Ligands for Peroxisome Proliferator-Activated Receptors α and δ, Proc. Natl. Acad. Sci. USA 94, 4312–4317.
Krey, G., Braissant, O., L'Horset, F., Kalkhoven, E., Perroud, M., Parker, M.G., and Wahli, W. (1997) Fatty Acids, Eicosanoids, and Hypolipidemic Agents Identified as Ligands of Peroxisome Proliferator-Activated Receptors by Coactivator-Dependent Receptor Ligand Assay, Mol. Endocrinol. 11, 779–791.
Forman, B.M., Chen, J., and Evans, R.M. (1996) The Peroxisome Proliferator-Activated Receptors: Ligands and Activators, Ann. N.Y. Acad. Sci. 804, 266–275.
Xu, H.E., Lambert, M.H., Montana, V.G., Parks, D.J., Blanchard, S.G., Brown, P.J., Sternbach, D.D., Lehmann, J.M., Wisely, G.B., Willson, T.M., et al. (1999) Molecular Recognition of Fatty Acids by Peroxisome Proliferator-Activated Receptors, Mol. Cell 3, 397–403.
Zomer, A.W.M., van der Burg, B., Jansen, G.A., Wanders, R.J.A., Poll-The, B.T., and van der Saag, P.T. (2000) Pristanic Acid and Phytanic Acid: Naturally Occurring Ligands for the Nuclear Receptor Peroxisome Proliferater-Activated Receptor α, J. Lipid Res. 41, 1801–1807.
Kliewer, S.A., Sundseth, S.S., Jones, S.A., Brown, P.J., Wisely, G.B., Koble, C.S., Devchand, P., Wahli, W., Willson, T.M., Lenhard, J.M., and Lehmann, J.M. (1997) Fatty Acids and Eicosanoids Regulate Gene Expression Through Direct Interactions with Peroxisome Proliferator-Activated Receptors α and γ, Proc. Natl. Acad. Sci. USA 94, 4318–4323.
Oliver, W.R., Shenk, J.L., Snaith, M.R., Russell, C.S., Plunket, K.D., Bodkin, N.L., Lewis, M.C., Winegar, D.A., Sznaidman, M.L., Lambert, M.H., et al. (2001) A Selective Peroxisome Prolierator-Activated Receptor Agonist Promotes Reverse Cholesterol Transport, Proc. Natl. Acad. Sci. USA 98, 5306–5311.
Forman, B.M., Tontonoz, P., Chen, J., Brun, R.P., Spiegelman, B.M., and Evans, R.M. (1995) 15-Deoxy-Δ12,14-Prostaglandin J 2 Is a Lignad for the Adipocyte Determination Factor PPARγ, Cell 83, 803–812.
Kliewer, S.A., Lenhard, J.M., Willson, T.M., Patel, I., Morris, D.C., and Lehmann, J.M. (1995) A Prostaglandin J2 Metabolite Binds Peroxisome Proliferator-Activated Receptor γ and Promotes Adipocyte Differentiation, Cell 83, 813–819.
Lehmann, J.M., Moore, L.B., Smith-Oliver, T.A., Wilkinson, W.O., Willson, T.M., and Kliewer, S.A. (1995) An Antidiabetic Thiazolidinedione Is a High Affinity Ligand for Peroxisome Proliferator-Activated Receptor γ (PPARγ), J. Biol. Chem. 270, 12953–12956.
Bergseth, S., and Bremer, J. (1990) Alkylthioacetic Acids (3-thia fatty acids) Are Metabolized and Excreted As Shortened Dicarboxylic-Acids in vivo, Biochim. Biophys. Acta 1044, 237–242.
Hvattum, E., Bergseth, S., Pedersen, C.N., Bremer, J., Aarsland, A., and Berge, RK. (1991) Microsomal Oxidation of Dodecylthioacetic Acid (a 3-thia fatty acid) in Rat Liver, Biochem. Pharmacol. 41, 945–953.
Berge, RK., Aarsland, A., Kryvi, H., Bremer, J., and Aarsaether, N. (1989) Alkylthioacetic Acid (3-thia fatty acids)—A New Group of Non-β-oxidizable, Peroxisome-Inducing Fatty Acid Analogues. I. A Study on the Structural Requirements for Proliferation of Peroxisomes and Mitochondria in Rat Liver, Biochim. Biophys. Acta 1004, 345–356.
Norrheim, L., Sørensen, H., Gautvik, K.M., Bremer, J., and Spydevold, Ø. (1990) Synergistic Actions of Tetradecylthioacetic Acid (TTA) and Dexamethasone on Induction of the Peroxisomal β-Oxidation and on Growth Inhibition of Morris Hepatoma Cells. Both Effects Are Counteracted by Insulin, Biochim. Biophys. Acta 1051, 319–323.
Spydevold, O., and Bremer, J. (1989) Induction of Peroxisomal β-Oxidation in 7800 C1 Morris Hepatoma Cells in Steady State by Fatty Acids and Fatty Acid Analogues, Biochim. Biophys. Acta 1003, 72–79.
Larsen, L.N., Bremer, J., Flock, S., and Skattebol, L. (1998) α- and β-Alkyl-Substituted Eicosapentaenoic Acids: Incorporation into Phospholipids and Effects on Prostaglandin H Synthase and 5-Lipoxygenase, Biochem. Pharmacol. 55, 405–411.
Madsen, L., Guerre-Millo, M., Flindt, E.N., Berge, K., Tronstad, K.J., Bergene, E., Sebokova, E., Rustan, A.C., Jensen, J., Mandrup, S., et al. (2002) Tetradecylthioacetic Acid Prevents High Fat Diet Induced Adiposity and Insulin Resistance, J. Lipid Res. 43, 742–750.
Jansen, G.A., Ofman, R., Ferdinandusse, S., Ijlst, L., Muijsers, A.O., Skjeldal, O.H., Stokke, O., Jakobs, C., Besley, G.T., Wraith, J.E., and Wanders, R.J. (1997) Refsum Disease Is Caused by Mutations in the Phytanoyl-CoA Hydroxylase Gene, Nat. Genet. 17, 190–193.
Wanders, R.J.A., and Tager, J.M. (1998) Lipid Metabolism in Peroxisomes in Relation to Human Disease, Mol. Aspects Med. 19, 69–154.
Chin, S.F., Liu, W., Storkson, J.M., Ha, Y.L., and Pariza, M.W. (1992) Dietary Sources of Conjugated Dienoic Isomers of Linoleic Acid, a Newly Recognized Class of Anticarcinogens, J. Food Compos. Anal. 5, 185–197.
Park, Y., Storkson, J.M., Albright, K.J., Liu, W., and Pariza, M.W. (1999) Evidence That the trans-10,cis-12 Isomer of Conjugated Linoleic Acid Induces Body Composition Changes in Mice, Lipids 34, 235–241.
Granlund, L., Juvet, L.K., Pedersen, J.I., and Nebb, H.I. (2003) Trans 10,cis 12-Conjugated Linoleic Acid Prevents Triacylglycerol Accumulation in Adipocytes by Acting as a PPARγ Modulator, J. Lipid Res. 44, 1441–1452.
Granlund, L., Larsen, L.N., Nebb., H.I., and Pedersen, J.I., Effects of Structural Changes of Fatty Acids on Lipid Accumulation in Adipocytes and Primary Hepatocytes, Biochim. Biophys. Acta, in press.
Jie, M.S.F.L., Pasha, M.K., and Alam, M.S. (1997) Synthesis and Nuclear Magnetic Resonance Properties of All Geometrical Isomers of Conjugated Linoleic Acids, Lipids 32, 1041–1044.
Lie Ken Jie, M.S.F., Lam, C.N.W., Ho, J.C.M., and Lau, M.M.L. (2003) Epoxidation of a Conjugated Linoleic Acid Isomer, Eur. J. Lipid Sci. Technol. 105, 391–396.
Lie Ken Jie, M.S.F., Pasha, M.K., and Alam, M.S. (1999) Nuclear Magnetic Resonance Spectroscopic Analysis of Conjugated Linoleic Acid Esters, in Advances in Conjugated Linoleic Acid Research, Volume 1 (Yurawecz, M.P., Mossoba, M.M., Kramer, J.K.G., Pariza, M.W., and Nelson, G.J., eds.) pp. 152–163, AOCS Press, Champaign.
Chen, C.-A., and Sih, C.J. (1998) Chemoenzymatic Synthesis of Conjugated Linoleic Acid, J. Org. Chem. 63, 9620–9621.
Chen, C.A., Lu, W., and Sih, C.J. (1999) Synthesis of 9Z,11E-Octadecadienoic and 10E,12Z-Octadecadienoic Acids, the Major Components of Conjugated Linoleic Acid, Lipids 34, 879–884.
Richardson, U.I., Snodgrass, P.J., Nuzum, C.T., and Tashjian, A.H., Jr. (1974) Establishment of a Clonal Strain of Hepatoma Cells Which Maintain in Culture the Five Enzymes of the Urea Cycle, J. Cell. Physiol. 83, 141–149.
Steineger, H.H., Sørensen, H.N., Tugwood, J.D., Skrede, S., Spydevold, O., and Gautvik, K.M. (1994) Dexamethasone and Insulin Demonstrate Marked and Opposite Regulation of the Steady-State mRNA Level of the Peroxisomal Proliferator-Activated Receptor (PPAR) in Hepatic Cells. Hormonal Modulation of Fatty-Acid-Induced Transcription, Eur. J. Biochem. 225, 967–974.
Sørensen, H.N., Hvattum, E., Paulssen, E.J., Gautvik, K.M., Bremer, J., and Spydevold, O. (1993) Induction of Peroxisomal Acyl-CoA Oxidase by 3-Thia Fatty Acid, in Hepatoma Cells and Hepatocytes in Culture Is Modified by Dexamethasone and Insulin, Biochim. Biophys. Acta 1171, 263–271.
Sørensen, H.N., Gautvik, K.M., Bremer, J., and Spydevold, O. (1992) Induction of the Three Peroxisomal β-Oxidation Enzymes Is Synergistically Regulated by Dexamethasone and Fatty Acids, and Counteracted by Insulin in Morris 7800C1 Hepatoma Cells in Culture, Eur. J. Biochem. 208, 705–711.
Wu, P., Skrede, S., Hvattum, E., and Bremer, J. (1993) Substrate and Hormone Regulation of Palmitoyl-CoA Synthetase in 7800 C1Morris Hepatoma Cells and Cultured Rat Hepatocytes, Biochim. Biophys. Acta 1170, 118–124.
Lewis, D.F.V., Jacobs, M.N., Dickins, M., and Lake, B.G. (2002) Molecular Modelling of the Peroxisome Proliferator-Activated Receptor α (PPARα) from Human, Rat and Mouse, Based on Homology with the Human PPARγ Crystal Structure, Toxicol. in Vitro 16, 275–280.
Berge, R.K., Aarsland, A., Kryvi, H., Bremer, J., and Aarsaether, N. (1989) Alkylthio Acetic Acids (3-thia fatty acids)—A New Group of Non-β-oxidizable Peroxosome-Inducing Fatty Acid Analogues—II. Dose-Response Studies on Hepatic Peroxisomal- and Mitochondrial Changes and Long-Chain Fatty Acid Metabolizing Enzymes in Rats, Biochem. Pharmacol. 38, 3969–3979.
Frøyland, L., Madsen, L., Sjursen, W., Garras, A., Lie, Ø., Songstad, J., Rustan, A.C., and Berge, R.K. (1997) Effect of 3-Thia Fatty Acids on the Lipid Composition of Rat Liver, Lipoproteins, and Heart, J. Lipid Res. 38, 1522–1534.
Madsen, L., Garras, A., Asins, G., Serra, D., Hegardt, F.G., and Berge, R.K. (1999) Mitochondrial 3-Hydroxy-3-methylglutaryl Conenzyme A Synthase and Carnitine Palmitoyltransferase II as Potential Control Sites for Ketogenesis During Mitochondrion and Peroxisome Proliferation, Biochem. Pharmacol. 57, 1011–1019.
Cronet, P., Petersen, J.F.W., Folmer, R., Blomberg, N., Sjøblom, K., Karlsson, U., Lindstedt, E., and Bamberg, K. (2001) Structure of the PPARα and-γ Ligand Binding Domain in Complex with AZ 242: Ligand Selectivity and Agonist Activation in the PPAR Family, Structure 9, 699–706.
Lewis, D.F.V., and Lake, B.G. (1998) Molecular Modelling of the Rat Peroxisome Proliferator-Activated Receptor-α (rPPARα) by Homology with Human Retinoic Acid X Receptor α (hRXRα) and Investigation of Ligand Binding Interactions: QSARs, Toxicol, in Vitro 12, 619–632.
Vaagenes, H., Madsen, L., Dyrøy, E., Elholm, M., Stray-Pedersen, A., Frøyland, L., Lie, Ø., and Berge, R.K. (1999) Methylated Eicosapentaenoic Acid and Tetradecylthioacetic Acid: Effects on Fatty Acid Metabolism, Biochem. Pharmacol. 58, 1133–1143.
Aarsland, A., Aarsaether, N., Bremer, J., and Berge, R.K. (1989) Alkylthioacetic Acids (3-thia fatty acids) as Non-β-oxidizable Fatty Acid Analogues: A New Group of Hypolipidemic Drugs. III. Dissociation of Cholesterol- and Triglyceride-Lowering Effects and The Induction of Peroxisomal β-Oxidation, J. Lipid Res. 30, 1711–1718.
Asiedu, D.K., Skorve, J., Willumsen, N., Demoz, A., and Berge, R.K. (1993) Early Effects on Mitochondrial and Peroxisomal β-Oxidation by the Hypolipidemic 3-Thia Fatty Acids in Rat Livers, Biochim. Biophys. Acta. 1166, 73–76.
Skorve, J., Asiedu, D., Rustan, A.C., Drevon, C.A., al-Shurbaji, A., and Berge, R.K. (1990) Regulation of Fatty Acid Oxidation and Triglyceride and Phospholipid Metabolism by Hypolipidemic Sulfur-Substituted Fatty Acid Analogues, J. Lipid Res. 31 1627–1635.
Willumsen, N., Vaagenes, H., Holmsen, H., and Berge, R.K. (1998) On the Effect of 2-Deuterium- and 2-Methyl-eicosapentaenoic Acid Derivatives on Triglycerides, Peroxisomal β-Oxidation and Platelet Aggregation in Rats, Biochim. Biophys. Acta. 1369, 193–203.
Wolfrum, C., Ellinghaus, P., Fobker, M., Seedorf, U., Assmann, G., Børchers, T., and Spener, F. (1999) Phytanic Acid Is Ligand and Transcriptional Activator of Murine Liver Fatty Acid Binding Protein, J. Lipid Res. 40, 708–714.
Norris, A.W., and Spector, A.A. (2002) Very Long Chain n−3 and n−6 Polyunsaturated Fatty Acids Bind Strongly to Liver Fatty Acid-Binding Protein, J. Lipid Res. 43, 646–653.
Huang, H., Starodub, O., McIntosh, A., Kier, A.B., and Schroeder, F. (2002) Liver Fatty Acid-Binding Protein Targets Fatty Acids to the Nucleus. Real Time Confocal and Multiphoton Fluorescence Imaging in Living Cells, J. Biol. Chem. 277, 29139–29151.
Wolfrum, C., Bormann, C.M., Borchers, T., and Spener, F. (2001) Fatty Acids and Hypolipidemic Drugs Regulate Peroxisome Proliferator-Activated Receptors α- and γ-Mediated Gene Expression via Liver Fatty Acid Binding Protein: A Signaling Path to the Nucleus, Proc. Natl. Acad. Sci. USA 98, 2323–2328.
Jia, Y., Qi, C., Kashireddy, P. Surapureddi, S., Zhu, Y., Rao, M.S., Roith, D., Chambon, P., Gonzalez, F.J., and Reddi, J.K. (2004) Transcription Coactivator PBP, the Peroxisome Proliferator-Activated Receptor (PPAR)-Binding Protein, Is Required for PPARα-Regulated Gene Expression in Liver, J. Biol. Chem. 279, 24427–24434.
Stanley, T.B. Leesnitzer, L.M., Montana, V.G., Galardi, C.M., Lambert, M.H., Holt, J.A., Xy, H.E., Moore, L.B., Blanchard, S.G., and Stimmel, J.B. (2003) Subtype Specific Effects of Peroxisome Proliferator-Activated Receptor Ligands on Corepressor Affinity, Biochemistry 42, 9278–9287.
Singh, H., Beckman, K., and Poulos, A. (1994) Peroxisomal β-Oxidation of Branched Chain Fatty Acids in Rat Liver. Evidence That Carnitine Palmitoyltransferase I Prevents Transport of Branched Chain Fatty Acids into Mitochondria, J. Biol. Chem. 269, 9514–9520.
Van Velhoven, P.P., Vanhoven, G., Vanhoutte, F., Dacremont, G., Parmentier, G., Eyssen, H.J., and Mannaerts, G.P. (1991) Identification and Purification of a Peroxisomal Branched Chain Fatty Acyl-Coa Oxidase, J. Biol. Chem. 266, 24676–24683.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Larsen, L.N., Granlund, L., Holmeide, A.K. et al. Sulfur-substituted and α-methylated fatty acids as peroxisome proliferator-activated receptor activators. Lipids 40, 49–57 (2005). https://doi.org/10.1007/s11745-005-1359-3
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11745-005-1359-3