Abstract
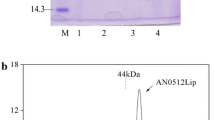
An extracellular 1,3-specific lipase with molecular weight of 35.5 kDa and an isoelectric point of 4.4 from Aspergillus niger has been purified 50-fold by pH precipitation followed by a series of chromatographic steps with an overall yield of 10%. The enzyme was homogeneous as judged by denaturing polyacrylamide gel electrophoresis and size-exclusion fast-performance liquid chromatography. It contained 2.8% sugar which was completely removed by endoglycosidase F treatment, and the deglycosylated enzyme retained full activity. The native lipase showed optimal activity between temperatures 35 and 55°C and pH 5.0 and 6.0. The amino acid composition and the N-terminal sequence were found to be different from lipases previously purified from A. niger. The enzyme was resistant to trypsin, chymotrypsin, endoprotease Glu-C, thrombin, and papain under native conditions but was susceptible to cleavage by the same proteases when heat-denatured.
Similar content being viewed by others
Abbreviations
- BSA:
-
bovine serum albumin
- DMSO:
-
dimethylsulfoxide
- FPLC:
-
fast-performance liquid chromatography
- HPLC:
-
high-performance liquid chromatography
- PAS:
-
periodate Schiff's stain
- PITC:
-
phenylisothio-cyanate
- PMSF:
-
phenylmethylsulfonylfluoride
- SDS-PAGE:
-
polyacrylamide gel electrophoresis in sodium dodecyl sulfate
- TCA:
-
trichloroacetic acid
- TLC:
-
thin-layer chromatography
References
Sarda, L., and Desnuelle, P. (1958) Action de la Lipase Pancre-atique sur les Esters en Emulsion, Biochim. Biophys. Acta 30, 513–520.
Derewanda, Z.S., and Sharp, A.M. (1993) News from the Inter-face: the Molecular Structures of Triglyceride Lipases, TIBS 18, 20–25.
Derewanda, Z.S. (1995) A Twist in the Tale of Lipolytic Enzymes, Nat. Struct. Biol. 2, 347–349.
Desnuelle, P. (1972) The Lipases, in The Enzymes (Boyer, P.D., ed.), pp. 575–616, Academic Press, New York.
Aires-Barros, M.R., Angela Taipa, M., and Cabral, J.M.S. (1994) Isolation and Purification of Lipases, in Lipases, Their Structure, Biochemistry, and Application (Woolley, P., and Peterson, S.B., eds.), pp. 243–270, Cambridge University Press, Cambridge.
Vulfson, E.N. (1994) Industrial Applications of Lipases, in Lipases, Their Structure, Biochemistry and Application (Woolley, P., and Peterson, S.B., eds.), pp. 271–288, Cambridge University Press, Cambridge.
Veeraragavan, K., Colpitts, T., and Gibbs, B.F. (1990) Purification and Characterization of Two Distinct Lipases from Geotrichum candidum, Biochim. Biophys. Acta 1044, 26–33.
Nagaoka, K., and Yamada, Y. (1973) Purification of Mucor Lipases and Their Properties, Agric. Biol. Chem. 37, 2791–2796.
Fukumoto, J., Iwai, M., and Tsujisaka, Y. (1964) Purification and Crystallization of a Lipase Secreted by Aspergillus niger, J. Gen. Appl. Microbiol. 10, 257.
Tsujisaka, Y., Iwai, M., and Tominaga, Y. (1973) Purification, Crystallization, and Some Properties of Lipases from Geotrichum candidum, Agric. Biol. Chem. 37, 1457–1464.
Tomizuka, N., Ota, Y., and Yamada, K. (1966) Studies on Lipase from Candida cylindracea. Part 1 Purification and Properties, Agric. Biol. Chem. 30, 576–584.
Rubin, B. (1994) Grease Pit Chemistry Exposed, Nat. Struct. Biol. 1, 568–572.
Derewanda, U., Swenson, L., Green, R., Wei, Y., Dodson, G.G., Yamaguchi, S., Haas, M.J., and Derewanda, Z.S. (1994) An Unusual Buried Polar Cluster in a Family of Fungal Lipases, Nat. Struct. Biol. 1, 36–47.
Tombs, M.P., and Blake, G.G. (1982) Stability and Inhibition of Aspergillus and Rhizopus Lipases, Biochim. Biophys. Acta 700, 81–89.
Höfelmann, M. (1985) Isolation, Purification, and Characterization of Lipase Isozymes from a Technical Aspergillus niger Enzyme, J. Food Sci. 50, 1721–1725.
Sugihara, A., Shimada, Y., and Tominaga, Y. (1988) Purification and Characterization of Aspergillus niger Lipase, Agric. Biol. Chem. 52, 1591–1592.
Torossian, K., and Bell, A.W. (1991) Purification and Characterization of an Acid-Resistant Lipase from Aspergillus niger, Biotechnol. Appl. Biochem. 13, 205–211.
Bernardi, G. (1971) Chromatography of Proteins on Hydroxyapatite, Methods Enzymol. 22, 325–339.
Kwon, D.Y., and Rhee, J.S. (1986) A Simple and Rapid Colorimetric Method for Determination of Free Fatty Acids for Lipase Assay, J. Am. Oil Chem. Soc. 63, 89–92.
Kouker, G., and Jaeger, K. (1987) Specific and Sensitive Plate Assay for Bacterial Lipase, Appl. Environ. Microbiol. 53, 211–213.
Bradford, M.M. (1976) A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding, Anal. Biochem. 72, 248–254.
Laemmli, U.K. (1970) Cleavage of Structural Proteins During the Assembly of the Head of Bacteriophage T4, Nature 227, 680–685.
Butcher, L.A., and Tomkins, J.K. (1986) A Comparison of Silver Staining Methods for Detecting Proteins in Ultrathin Gels on Support Films After Isoelectric Focussing, Anal. Biochem. 148, 384–388.
Konat, G., Offner, H., and Mellah, J. (1984) Improved Sensitivity for Detection and Quantitation of Glycoproteins on Polyacrylamide Gels, Experientia 40, 303.
Thornton, D.J., Carlstedt, I., and Sheehan, J.K. (1994) Identification of Glycoproteins on Nitrocellulose Membranes and Gels, in Basic Protein and Peptide Protocols (Walker, J.M., ed.) pp. 119–128, Humana Press, New Jersey.
Sztajer, H., Lunsdorf, H., Erdmann, H., Menge, U., and Schmid, R.D. (1992) Purification and Properties of Lipase from Penicillium simplissimum, Biochim. Biophys. Acta 1124, 253–261.
Haas, M.J., Cichowicz, D.J., and Bailey, D.G. (1992) Purification and Characterization of an Extracellular Lipase from Rhizopus delemar, Lipids 27, 571–576.
Boel, E., Huge-Jensen, B., Christensen, M., Thim, L., and Fiil, N.P. (1988) Rhizomucor miehei Triglyceride Lipase Is Synthesized as a Precursor, Lipids 23, 701–706.
Maley, F., Trimble, R.B., Tarentino, A.L., and Plummer, T.H., Jr. (1989) Characterization of Glycoproteins and Their Associated Oligosaccharides Through the Use of endoglycosidases, Anal. Biochem. 180, 195–204.
Klibanov, A.M. (1989) Enzymatic Catalysis in Anhydrous Organic Solvents, Trends. Biochem. Sci. 14, 141–144.
Bott, R., Shield, J.W., and Poulose, A.J. (1994) Protein Engineering of Lipases, in Lipases, Their Structure, Biochemistry and Application (Woolley, P., and Peterson, S.B., eds.), pp. 337–354, Cambridge University Press, Cambridge.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Haridasan Namboodiri, V.M., Chattopadhyaya, R. Purification and biochemical characterization of a novel thermostable lipase from Aspergillus niger . Lipids 35, 495–502 (2000). https://doi.org/10.1007/s11745-000-549-3
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11745-000-549-3