Abstract
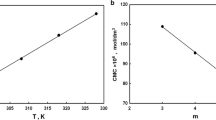
Three amidosulfobetaine surfactants were synthesized namely: 3-(N-pentadecanamidopropyl-N,N-dimethyl ammonium) propanesulfonate (2a); 3-(N-heptadecanamidopropyl-N,N-dimethyl ammonium) propanesulfonate (2b), and 3-(N-nonadecanamidopropyl-N,N-dimethyl ammonium) propanesulfonate (2c). These surfactants were prepared by direct amidation of commercially available fatty acids with 3-(dimethylamino)-1-propylamine and subsequent reaction with 1,3-propanesultone to obtain quaternary ammonium salts. The synthesized surfactants were characterized by IR, NMR and mass spectrometry. Thermogravimetric analysis (TGA) results showed that the synthesized surfactants have excellent thermal stability with no major thermal degradation below 300 °C. The critical micelle concentration (CMC) values of the surfactants 2a and 2b were found to be 2.2 × 10−4 and 1.04 × 10−4 mol/L, and the corresponding surface tension (γCMC) values were 33.14 and 34.89 mN m−1, respectively. The surfactants exhibit excellent surface properties, which are comparable with conventional surfactants. The intrinsic viscosity of surfactant (2b) was studied at various temperatures and concentrations of multi-component brine solution. The plot of natural logarithm of relative viscosity versus surfactant concentration obtained from Higiro et al. model best fit the surfactant behavior. Due to good salt resistance, excellent surface properties and thermal stability, the synthesized surfactant has potential to be used in various oil field applications such as enhanced oil recovery, fracturing, acid diversion, and well stimulation.






Similar content being viewed by others
References
Olajire AA (2014) Review of ASP EOR (alkaline surfactant polymer enhanced oil recovery) technology in the petroleum industry: prospects and challenges. Energy 77:963–982
Howe AM, Clarke A, Mitchell J, Staniland J, Hawkes L, Whalan C (2015) Visualising surfactant enhanced oil recovery. Colloids Surf A Physicochem Eng Asp 480:449–461
Kamal MS, Sultan AS, Hussein IA (2015) Screening of amphoteric and anionic surfactants for cEOR applications using a novel approach. Colloids Surf A: Physicochem Eng Asp 476:17–23
Kamal MS, Hussien IA, Sultan AS, Han M (2013) Rheological study on ATBS-AM copolymer-surfactant system in high-temperature and high-salinity environment. J Chem. doi:10.1155/2013/801570
Borhan N, Halim NH, Ibrahim BM, Mohamad J (2014) An investigation of micro-emulsion and fine foams induced by EOR application in Malaysian fields. In: International Petroleum Technology Conference, 2014. International Petroleum Technology Conference
Mohanty KK (2015) Synergy between nanoparticles and surfactants in stabilizing foams for oil recovery. Energy Fuels 29(2):467–479
Wang X, Wang R, Zheng Y, Sun L, Yu L, Jiao J, Wang R (2013) Interaction between zwitterionic surface activity ionic liquid and anionic surfactant: Na+ -driven wormlike micelles. J Phys Chem B 117(6):1886–1895
Bordes R, Tropsch J, Holmberg K (2009) Role of an amide bond for self-assembly of surfactants. Langmuir 26(5):3077–3083
Kefi S, Lee J, Pope T, Sullivan P, Nelson E, Hernandez A, Olsen T, Parlar M, Powers B, Roy A (2004) Expanding applications for viscoelastic surfactants. Oilfield Rev 16(4):10–23
Omeiza AA, Samsuri AB (2006) Viscoelastic surfactants application in hydraulic fracturing, it’s set back and mitigation—an overview. ARPN J Eng Appl Sci 9(1):25–29
Lakatos I, Toth J, Bodi T, Lakatos-Szabo J, Berger P, Lee C (2007) Application of viscoelastic surfactants as mobility-control agents in low-tension surfactant floods. In: International symposium on oilfield chemistry, 28 Feb–2 Mar, Houston, TX
Kamal MS, Sultan AS, Al-Mubaiyedh UA, Hussien IA, Pabon M (2014) Evaluation of rheological and thermal properties of a new fluorocarbon surfactant-polymer system for EOR applications in high-temperature and high-salinity oil reservoirs. J Surfactants Deterg 17(5):985–993
Mou J, Liu M, Zheng K, Zhang S (2015) Diversion conditions for viscoelastic-surfactant-based self-diversion acid in carbonate acidizing. SPE Prod Oper 30(02):121–129
Kamal MS, Sultan AS, Al-Mubaiyedh UA, Hussein IA, Feng Y (2015) Rheological properties of thermoviscosifying polymers in high-temperature and high-salinity environments. Can J Chem Eng 93(7):1194–1200. doi:10.1002/cjce.22204
Crews JB, Huang T (2008) Performance enhancements of viscoelastic surfactant stimulation fluids with nanoparticles. In: Europec/EAGE Conference and Exhibition, Society of Petroleum Engineers
Sultan A (2009) Molecular simulation study of diverting materials used in matrix acidizing. Texas A&M University, Texas
Xiaowu Y, Yiding S, Peizhi L (2010) Solution properties of anionic hydrophobic association polyacrylamide modified with fluorinated acrylate. J Polym Res 17(4):601–606
Chu Z, Feng Y (2009) A facile route towards the preparation of ultra-long-chain amidosulfobetaine surfactants. Synlett 16:2655–2658
Chu Z, Feng Y (2011) Empirical correlations between Krafft temperature and tail length for amidosulfobetaine surfactants in the presence of inorganic salt. Langmuir 28(2):1175–1181
Zaky MF, Badawi AM, El Sabbah I, Ghani RAA, Hendawy ME (2015) Synthesis, characterization and surface activities of cationic polysaccharide (Aloe) schiff base surfactants. J Surfactants Deterg 18(3):455–461
Dong Z, Zheng Y, Zhao J (2014) Synthesis, physico-chemical properties and enhanced oil recovery flooding evaluation of novel zwitterionic gemini surfactants. J Surfactants Deterg 17(6):1213–1222
Zhang Y, Zhou D, Feng Y (2015) In-situ formation of viscoelastic wormlike micelles in mixtures of non-surface-active compounds. J Surfactants Deterg 18(1):189–198
Dharaiya N, Patriati A, Kuperkar K, Putra E, Bahadur P (2012) Spectral and scattering microstructural investigation in cationic gemini surfactants (12-s-12) induced by p-toluidine. Colloids Surf A: Physicochem Eng Asp 396:1–7
Wang Y, Zhang Y, Liu X, Wang J, Wei L, Feng Y (2014) Effect of a hydrophilic head group on krafft temperature, surface activities and rheological behaviors of erucyl amidobetaines. J Surfactants Deterg 17(2):295–301
Chen K, Zhou X, Wang X (2014) Synthesis and application of a hyperbranched polyester quaternary ammonium surfactant. J Surfactants Deterg 17(6):1081–1088
Han Z, Yang X, Liu Y, Wang J, Gao Y (2015) Physicochemical properties and phase behavior of didecyldimethylammonium chloride/alkyl polyglycoside surfactant mixtures. J Surfactants Deterg 1–9. doi:10.1007/s11743-015-1679-5
Dam T, Engberts J, Karthäuser J, Karaborni S, Van Os N (1996) Synthesis, surface properties and oil solubilisation capacity of cationic gemini surfactants. Colloids Surf A: Physicochem Eng Asp 118(1):41–49
Wang L, Liu J, Huo S, Deng Q, Yan T, Ding L, Zhang C, Meng L, Lu Q (2014) Synthesis and surface properties of novel gemini imidazolium surfactants. J Surfactants Deterg 17(6):1107–1116
Song B, Hu X, Shui X, Cui Z, Wang Z (2016) A new type of renewable surfactants for enhanced oil recovery: dialkylpolyoxyethylene ether methyl carboxyl betaines. Colloids Surf A Physicochem Eng Asp 489:433–440
Geng XF, Hu XQ, Xia JJ, Jia XC (2013) Synthesis and surface activities of a novel di-hydroxyl-sulfate-betaine-type zwitterionic gemini surfactants. Appl Surf Sci 271:284–290
Hu S-S, Zhang L, Xu Z-C, Gong Q-T, Jin Z-Q, Luo L, Zhang L, Zhao S (2015) Wettability alteration by novel betaines at polymer–aqueous solution interfaces. Appl Surf Sci 355:868–877
Higiro J, Herald T, Alavi S (2006) Rheological study of xanthan and locust bean gum interaction in dilute solution. Food Res Int 39(2):165–175
Acknowledgments
This work was supported by King Abdulaziz City for Science and Technology (KACST) through the Science and Technology Unit at King Fahd University of Petroleum and Minerals (KFUPM) through Project No. 10-OIL1378-04 as part of the National Science Technology and Innovation Plan.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Shakil Hussain, S.M., Animashaun, M.A., Kamal, M.S. et al. Synthesis, Characterization and Surface Properties of Amidosulfobetaine Surfactants Bearing Odd-Number Hydrophobic Tail. J Surfact Deterg 19, 413–420 (2016). https://doi.org/10.1007/s11743-016-1788-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-016-1788-9