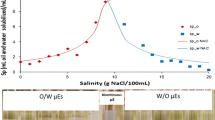
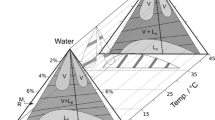
Abstract
Characterizing the hydrophilic-lipophilic nature of a surfactant molecule has been a challenge for colloid scientists and technologists. The hydrophilic-lipophilic balance (HLB), the packing factor, the phase inversion temperature (PIT) and the natural curvature of the surfactant are all terms that seek to address this issue. In this article we build on the hydrophilic–lipophilic difference concept (HLD) (Salager et al. Langmuir, 16, 5534–5539, 2000) to develop a methodology to determine a characteristic curvature (Cc) for ionic surfactants based on the phase behavior of mixed ionic surfactant microemulsions. In essence, the method consists of evaluating the shift in optimal electrolyte concentration as a function of the mole fraction of the test surfactant in a mixture with a reference surfactant, sodium dihexyl sulfosuccinate (SDHS) and applying the appropriate HLD equation for ionic surfactant mixtures to determine Cc. The values of Cc were determined for a range of surfactants, including sodium dodecyl sulfate (SDS), sodium dodecyl benzene sulfonate (SDBS), sodium naphthenate, and others. The method was also extrapolated to nonionic additives and hydrophilic linkers. It was observed that the calculated values of Cc were similar to those predicted by group contribution models, however the proposed method can be used even for complex surfactant mixtures. Finally, when Cc values were compared to apparent packing factor and HLB values, it was found that Cc is correlated with the apparent packing factor of ionic surfactants, and that Cc correlates with the HLB value for nonionic amphiphiles. The physical interpretation of Cc, and its potential application in the Net-Average Curvature equation of state for oil-surfactant-water systems is discussed.










Similar content being viewed by others
References
Scheibel JJ (2004) The evolution of anionic surfactant technology to meet the requirements of the laundry detergent industry. J Surfact Deterg 7:319
Khan A, Marques EF (1999) Synergism and polymorphism in mixed surfactant systems. Curr Opin Colloid Interface Sci 4:402
Rosen MJ (1986) Molecular interaction and synergism in mixed surfactant systems. ACS Symp Ser 311:144
Rosen MJ (2004) Surfactants and interfacial phenomenon in Wiley Interscience. John Wiley & Sons, Inc., Hoboken, pp 379–414
Holland PM, Rubingh DN (1992) Mixed surfactant systems. ACS Symp Ser 501:31
Shiloach A, Blankschtein D (1998) Predicting micellar solution properties of binary surfactant mixtures. Langmuir 14:1618–1636
Nishikido N (1990) Phase equilibria of nonionic surfactant-water systems as a function of temperature and pressure. Effect of mixing of surfactants. J Colloid Interface Sci 136:401
Rodriguez CH, Scamehorn JF (1999) Modification of krafft temperature or solubility of surfactants using surfactant mixtures. J Surfact Deterg 2:17
Shinoda K, Friberg S (1986) Emulsions and solubilization. Wiley, New York
Kunieda H, Yamagata M (1993) Mixing of nonionic surfactants at water–oil interfaces in microemulsions. Langmuir 9:3345
Yamaguchi S, Kunieda H (1997) Determination of a three-phase tie triangle (the hydrophile–lipophile balance plane) in a composition tetrahedron: evaluation of the composition of adsorbed mixed-surfactant and the monomeric solubilities of short-chain surfactant. Langmuir 13:6995
Rosen MJ, Wang H, Shen P, Zhu Y (2005) Ultralow interfacial tension for enhanced oil recovery at very low surfactant concentrations. Langmuir 21:3749
Israelachvili JN (1987) Physical principles of surfactant self-association into Micelles, bilayers, vesicles and microemulsion droplets. In: Mittal KL (ed) Surfactants in solution: recent developments, vol 4 Plenum 3–34
Mitchell J, Ninham B (1981) Micelles, vesicles and microemulsions. J Chem Soc Faraday Trans II 77:601
Nagarajan R, Ruckenstein E (2000) Molecular theory of microemulsions. Langmuir 16:6400
Becher Paul (1984) Hydrophile lipophile balance: history and recent developments. J Dispersion Sci Tech 5:81
Keggel WK, Overbeek JTh, Lekkerkerker HN (1999) Thermodynamics of microemulsions I. In: Kumar P, Mittal KL (eds) Handbook of microemulsion science and technology. Marcel Dekker, New York, pp 13–44
Kralchevsky PA, Ericksson JC, Ljunggren S (1994) Theory of curved interfaces and membranes: mechanical and thermodynamical approaches. Adv Colloid Interface Sci 48:19
Peck DG, Johnston KP (1993) Prediction of interfacial properties in microemulsions: the lattice fluid self-consistent field theory. J Phys Chem 97:5661
Acosta E, Szekeres E, Sabatini DA, Harwell JH (2003) Net-average curvature model for solubilization and supersolubilization in surfactant microemulsions. Langmuir 19:186
Salager JL, Morgan J, Schechter RS, Wade WH, Vasquez E (1979) Optimum formulation of surfactant-oil-water systems for minimum tension and phase behavior. Soc Petrol Eng J 19:107
Salager JL (1999) Ionic microemulsions. In: Kumar P, Mittal KL (eds) Handbook of microemulsion science and technology. Marcel Dekker, New York, pp 247–280
Salager JL, Márquez N, Graciaa A, Lachaise J (2000) Partitioning of ethoxylated octylphenol surfactants in microemulsion-oil-water systems: influence of temperature and relation between partitioning coefficient and physicochemical formulation. Langmuir 16:5534
Salager JL (1999) Microemulsions. In: Broze G (eds) Handbook of detergents—part a: properties. Marcel Dekker, New York, pp 253–302
Salager JL (1996) Quantifying the concept of physico-chemical formulation in surfactant-oil-water systems—state of the art. Prog Colloid Polym Sci 100:137–142
Bourrel M, Schecter R (1988) Microemulsions and related systems. Marcel Dekker, New York
Salager JL, Anton RE, Anderez J, Aubry JM (2001) Formulation des microémulsions par la méthode HLD. Techniques de l’Ingenieur, Genie des Procedes J2 157:1
Baran JR, Pope GA, Wade WH, Weerasooriya V, Yapa A (1994) Microemulsion formation with mixed chlorinated hydrocarbon liquids. J Colloid Interface Sci 168:67
Bellocq AM (1999) In: Kumar P, Mittal KL (eds) Handbook of microemulsion science and technology. Marcel Dekker, New York, p 139
Witthayapanyanon A, Acosta EJ, Harwell JH, Sabatini DA (2006) Formulation of ultralow interfacial tension systems using extended surfactants. J Surfact Deterg 9:331
Acosta E, Uchiyama H, Sabatini DA, Harwell JH (2002) The role of hydrophilic linkers. J Surfact Deterg 5:151
Saab J, Mokbel I, Razzouk AC, Ainous N, Zydowicz N Jose J (2005) Quantitative extraction procedure of naphthenic acids contained in crude oils. Characterization with different spectroscopic methods. Energy Fuels 19:525
Havre TE, Sjoblom J, Vindstad JE (2003) Oil/water-partitioning and interfacial behavior of naphthenic acids. J Dispersion Sci Technol 24:789
Li Z, Cranston B, Zhao L, Choi P (2005) Molecular dynamics studies of the stability of water/n-heptane interfaces with adsorbed naphthenic acids. J Phys Chem B 109:20929
De Gennes PG, Taupin C (1982) Microemulsions and the flexibility of oil/water interfaces. J Phys Chem 86:2294
Acosta E, Tran S, Uchiyama H, Sabatini DA, Harwell JH (2002) Formulating chlorinated hydrocarbon microemulsions using linker molecules. Environ Sci Technol 36:4618
Acosta E (2004) Modeling and formulation of microemulsions: the net-average curvature model and the combined linker effect. Dissertation. University of Oklahoma, Norman, pp 308
Uchiyama H, Acosta E, Tran S, Sabatini DA, Harwell JH (2000) Supersolubilization in chlorinated hydrocarbon microemulsions: solubilization enhancement by lipophilic and hydrophilic linkers. Ind Eng Chem Res 39:2704
Acosta EJ, Harwell JH, Sabatini DA (2004) Self-assembly in linker-modified microemulsions. J Colloid Interface Sci 274:652
Griffin WC (1949) Classification of surface active agents by HLB. J Soc Cosmet Chem 1:311–326
Davies JT (1957) A quantitative kinetic theory the emulsion type. I. Physical chemistry of the emulsifying agent, in gas/liquid and liquid/liquid interfaces. In: Proceedings of the second international congress on surface activity, Butterworth, London, vol 1, p 426
Israelachvili JN (1992) Intermolecular and surface forces, 2nd edn. Academic Press, New York
Tanford C (1980) The hydrophobic effect. 2nd edn, Wiley, New York, p 52
Maitra A (1984) Determination of size parameters of water-aerosol OT-oil reverse micelles from their nuclear magnetic resonance data. J Phys Chem 88:5122–5125
Shinoda K, Takeda H (1970) The effect of added salts in water on the hydrophile–lipophile balance of nonionic surfactants: the effect of added salts on the phase inversion temperature of emulsions. J Colloid Interface Sci 32:642
Smith DH (1985) The role of critical points and binodal surfaces in emulsion type, HLB, and the phase inversion temperature: evidence from the cyclohexane/water/i-C9H19C6H4 (OC2H4)9.20H/temperature diagram. J Colloid Interface Sci 108:471
Binks BP, Cho W-G, Fletcher PDI, Petsev DN (2000) Stability of oil-in-water emulsions in a low interfacial tension system. Langmuir 16:1025
Hwan R-N, Miller CA, Fort T (1979) Determination of microemulsion phase continuity and drop size by ultracentrifugation. J Colloid Interface Sci 68:221
Acknowledgments
This work was partially supported by the Canada Foundation and Innovation, The Connaught Research Foundation of the University of Toronto, The natural science and engineering research council of Canada (NSERC), and a University of Toronto Open Fellowship to A. Bhakta and Jessica S. Yuan. We are grateful to Professor David A. Sabatini and his group at University of Oklahoma for their assistance with the gas chromatography measurements.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Acosta, E.J., Yuan, J.S. & Bhakta, A.S. The Characteristic Curvature of Ionic Surfactants. J Surfact Deterg 11, 145–158 (2008). https://doi.org/10.1007/s11743-008-1065-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-008-1065-7