Abstract
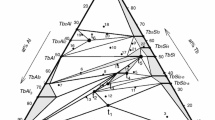
Nine limiting binaries of Al2O3-SiO2-Re2O3(Re=Nd, Sm, Gd and La) system are assessed. The binary diagrams or experimental information from Toropov, Mizuno, Aramaki, Bondar, Rolin and Coutures are optimized with the substitutional model of Kaufman and Nesor and the approximate formula of fusion free energy for rare earth element oxides of Wu and Pelton. The extracted Gibbs free energies of pure solid oxides and stoichiometric phases and the solution parameters are used to estimate the corresponding binaries, liquidus surfaces and a series of isothermal sections of four ternaries Al2O3-SiO2-Nd2O3, Al2O3-SiO2-Sm2O3, Al2O3-SiO2-Gd2O3, and Al2O3-SiO2-La2O3. In the Al2O3-SiO2-Gd2O3 system samples as fired at exact temperature with different compositions were analyzed by X-ray diffractometer and the detected results are fitted with the calculation of isothermal sections.
Similar content being viewed by others
Abbreviations
- G φ :
-
Gibbs free energy of φ Phase
- °G φ i :
-
Gibbs free energy of pure component i of φ phase
- X i :
-
mole fraction of component i
- E G φ :
-
excess free energy of φ phase
- k j :
-
j-th order parameter of Redlich-Kister polynomial
- ΔGf(i) :
-
fusion free energy of component i
- ΔHf(i) :
-
fusion enthalpy of component i
- ΔSf(i) :
-
fusion entropy of component i
- °G si :
-
Gibbs free energy of pure component i of solid phase
- °G l-Al 2O3 :
-
Gibbs free energy of pure A12O3 of liquid phase
- °G s-Al 2O3 :
-
Gibbs free energy of pure AI2O3 of solid phase
- G(La2Oe)0.333(SiO2)0.6667:
-
Gibbs free energy of stoichiometric phase (La2O3)0.333(SiO2)0.6667
- k31-(Sm2O3)(Al2O3):
-
3th order solution parameter of Redlich-Kister polynomial in Sm2O3 and Al2O3 binary
References
Li C., Bulletin of Ceramics, 1987, 10: 34
Erbe E. M. & Day D. E., J. Am. Ceram. Soc., 1990, 73: 2708–2713
Kohli J. T. & Shely J. E., Phys. Chem. Glasses, 1991, 32: 67
Hyatt M. J. & Day D. E., Am. Ceram. Soc., 1987, 70: c283-c287
Hirosaki N., Okada A. & Matoba K., J. Am. Ceram . Soc, 1988, 71:cl44-cl47
Cinibulk M. K. & Thomas G., J. Am. Ceram. Soc., 1992, 75: 2037–2043
Kaufman L. & Nesor H. CALPHAD, 1978, 2: 35–53
Hillert M. & Staffansson L. I., Acta Chemica Scandinavica, 1970,24: 3618–3626
Sundman B. & Agren J., J. Phys. Chem. Solids, 1981, 42: 297–301
Pelton A. D. & Blander M., Metall. Trans. B, 1986, 17:805–815
Wu P. & Pelton A. D., J. Alloys Compounds, 1992, 179:259–287
Hillert M. & Jonsson S., CALPHAD, 1992, 16: 193–198
Hillert M. & Jonsson S., CALPHAD, 1992, 16: 199–205
Du Y., Jin Z. & Huang P., CALPHAD, 1992, 16: 221–230
Lukas H. L., Henig E. Th. & Zimmermann B., CALPHAD, 1977, 1: 225–236
Aramaki S. & Roy R., J. Am. Ceram . Soc., 1962, 45: 229–242
Klug F.J. & Pask S.P., J. Am. Ceram. Soc., 1987, 70: 750–759
Huang J. G. & Li W.D., Bulletin of Ceramics, 1993, 6: 50–54
Li L., Sun W., Wang P. & Tang Z., Phys. Chem. Glasses, 1997, 38(6): 323–326
Coutures J.P., J. Am. Ceram. Soc., 1985, 68(3): 105–107
Toropov N. A., Trans. Inter. Ceram. Congr., 7th, London, 1960: 440
Bondar A. and Toropov N. A., Bull. Acad. Sci. USSR Div. Chem. Sci., 1966: 195
Toropov N. A. and Bondar A., Bull. Acad. Sci. USSR Div. Chem. Sci., 1966: 1279
Toropov N. A., Trans. Inter. Ceram. Congr., 7th, London, 1960: 441
Felsche J., Structure and Bonding, 1973, 13: 99
Kolitsch U., Seifert H. J., Aldinger F., J. Alloys and Compounds, 1997, 257:104
Bondar I. A., Ceram. Int., 1973,13: 99
Mizuno M., Yamada T. & Noguchi T., Yogyo-Kyokai-Shi, 1977, 85(11): 543–548
Budnikov P. P., Kushakovskii V. I., & Belevantsev V. S., Dokl. Akad. NaukSSSR, 1965, 165(5): 1077
Shishido T., J. Mat. Sci., 1979, 14: 823–830
Wang X. H., Lejus A. M. & Vivien D., J. Am. Ceram. Soc., 1990, 73: 770–774
Wu P. and Pelton A. D., J. Alloy and Compounds, 1992, 179:259
Bondar I. A. and Vinogradova N. V., Izv. Akad. Nauk SSSR, Ser. Khim., 1964, 5: 785–786
Fritsche E. T. and Tensmeyer L. G., J. Am. Ceram. Soc., 1967, 50(3):167–168
Rolin M. and Thanh P. H., Rev. Hautes Temp. Refract., 1965, 2: 182
Mizuno M., Berjoan R., Coutures J. P. and Foex M., Yogyo-Kyokai-Shi, 1974, 82(12): 631–636
Yamaguchi O., Sugiura K., Mitsui A. and Shimizu K., J. Am. Ceram. Soc., 1985, 68: c44-c45
Toropov N. A., Bondar I. A. and Galahov F. Ya., Trans. Inter. Ceram . Congr., 8th, Copenhagen, 1962: 87; Toropov N. A. and Bondar I. A., Izv. Akad. Nauk SSSR, Otd. Khim., 1961, 5: 740
Sun G., Li W. & Wang J., Chin. Rare-earth Element, 1991, 9(2): 128
Shevthenko A. V. & Lopato L. M., Thermochem. Acta, 1985, 93: 537
Li L., Sun W., Wang P. & Tang Z., J. Chin. Rare-earth Element, 1998, 16: 483–489
Li L., Sun W., Wang P. & Tang Z., Phys. Chem. Glasses, 1999, 40: 126–129
Li L., Sun W., Wang P. & Tang Z., J. Mat. Sci. & Tec, 1999, 15(4):1–5
Author information
Authors and Affiliations
Additional information
Supported by State Key Lab of High Performance Ceramics and Superfine Micro-structure (9517 and 9708)
About this article
Cite this article
Li, L., Tang, Zj., Sun, Wy. et al. Phase diagram estimation of the Al2O3-SiO2-Re2O3 systems. J. of Shanghai Univ. 4, 72–80 (2000). https://doi.org/10.1007/s11741-000-0036-7
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s11741-000-0036-7