Abstract
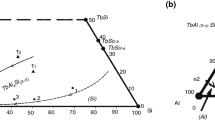
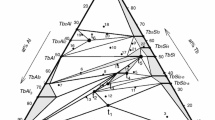
In the framework of a systematic investigation of intermetallic systems, constituted by aluminium and silicon with a rare earth metal, the isothermal section at 500 °C of the Pr–Al–Si system has been experimentally investigated. The experimental techniques used were scanning electron microscopy, electron microprobe analysis and X-ray powder diffraction, and some samples have been analysed by differential thermal analysis. The existence of six ternary compounds has been confirmed, one of them showing a composition homogeneity range: τ1 PrAl2Si2 (hP5-Ce2SO2 type), τ2 Pr3Al4Si6 (hP13-Ce3Al4Si6 type), τ3 PrAlSi2 (hP8-CeAlSi2 type), τ4 Pr2Al3Si (hP3-AlB2 type), τ5 PrAl(1−x)Si(1+x) (tI12-αThSi2Si type) and τ6 Pr2AlSi (oS8-CrB type). A few compounds pertaining to the binary boundary systems Pr–Al and Pr–Si dissolve the third element.




Similar content being viewed by others
References
Miller WS, Zhuang L, Bottema J, Wittebrood AJ, De Pret P, Haszler A, Vieregge A. Recent development in aluminium alloys for the automotive industry. Mater Sci Eng, A. 2000;280:37–49.
Chen CL, Richter A, Thomson RC. Mechanical properties of intermetallic phases in multi-component Al–Si alloys using nanoindentation. Intermetallics. 2009;17:634–41.
Ye HJ. An overview of the development of Al–Si-alloy based material for engine applications. J Mater Eng Perform. 2003;12:288–97.
Zhu M, Jian Z, Yao L, Liu C, Yang G, Zhou Y. Effect of mischmetal modification treatment on the microstructure, tensile properties, and fracture behavior of Al–7.0%Si–0.3%Mg foundry aluminum alloys. J Mater Sci. 2011;46:2685–94.
Qiu H, Yan H, Hu Z. Effect of samarium (Pr) addition on the microstructures and mechanical properties of Al–7Si–0.7 Mg alloys. J Alloys Compd. 2013;567:77–81.
Mazahery A, Shabani MO. Modification mechanism and microstructural characteristics of eutectic Si in casting Al–Si alloys: a review on experimental and numerical studies. J Miner Met Mater Soc. 2014;166(5):726–38.
Heusler L, Schneider W. Influence of alloying elements on the thermal analysis results of Al–Si cast alloys. J Light Met. 2002;2:17–26.
Nogita K, Yasuda H, Yoshiya M, McDonald SD, Uesugi K, Tacheuchi A, Suzuki Y. The role of trace element segregation in the eutectic modification of hypoeutectic Al–Si alloys. J Alloys Compd. 2010;489:415–20.
Cardinale AM, Macciò D, Delfino S, Saccone A. Experimental investigation of the Nd–Al–Si system. J Therm Anal Calorim. 2011;103:103–9.
Cardinale AM, Macciò D, Delfino S, Saccone A. Phase equilibria of the Dy–Al–Si system at 500°C. J Therm Anal Calorim. 2012;108:817–23.
Cardinale AM, Macciò D, Delfino S, Saccone A. Phase equilibria in the Sm–Al–Si system at 500°C. J Therm Anal Calorim. 2014;116:61–7.
Murray JL, McAlister AJ. The Al–Si (aluminum–silicon) system. Bull Alloy Phase Diagr. 1984;5:74–84.
Okamoto H. Desk handbook: phase diagrams for binary alloys. Materials Park: ASM Internatinal; 2000.
Jin LL, Kang YB, Chartrand P, Fuerst CD. Thermodynamic evaluation and optimization of Al–La, Al–Ce, Al–Pr, Al–Nd and Al–Pr systems using the modified quasichemical model for liquids. CALPHAD Comput Coupling Phase Diagr Thermochem. 2011;35:30–41.
Nakonechna N, Lyaskovska N, Romaniv O, Starodub P, Gladyshevskii E. Pr–Al–Si phase diagram (0–0.33 at.fract. Pr) and crystal structure of the compounds. Visn Lviv Univ Ser Khim. 2001;40:61–7.
Muts N, Gladyshevskii RE, Gladyshevskii EI. Crystal structures of the compounds PrAl2Si2, Pr3Al4Si6 and PrAlSi2. J Alloys Compd. 2005;402:66–9.
Bobev S, Tobash PH, Fritsch V, Thompson JD, Hundley MF, Sarrao JL, Fisk Z. Ternary rare-earth alumo-silicides—single-crystal growth from Al flux, structural and physical properties. J Solid State Chem. 2005;178:2091–103.
Lyaskovska N, Romaniv O, Semus’o N, Gladyshevskii E. Crystal structures of the compounds RAl0.5−xSi0.5+x (R = La, Ce, Pr, Nd, Pr, Gd), R3Al4Si6 (R = La, Pr), and RAlSi2 (R = Pr, Nd). J Alloys Compd. 2004;367:180–4.
Kraus W, Nolze G. Powder cell for windows. Berlin: Federal Institute for Materials Research and Testing; 1999.
King G, Schwarzenbach D. Latcon. In: Hall SR, Du Boulay DJ, Olthof-Hazekamp R, editors. Xtal3.7 system. Perth: University of Western Australia; 2000.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cardinale, A.M., Macciò, D. & Saccone, A. Phase relationships of the R–Al–Si systems. J Therm Anal Calorim 121, 1151–1157 (2015). https://doi.org/10.1007/s10973-015-4906-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4906-4