Abstract
Introduction
The mRNA vaccines mRNA-1273 and BNT162b2 demonstrated high efficacy against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in phase 3 clinical trials, including among older adults. To inform coronavirus disease 2019 (COVID-19) vaccine selection, this systematic literature review (SLR) and meta-analysis assessed the comparative effectiveness of mRNA-1273 versus BNT162b2 in older adults.
Methods
We systematically searched for relevant studies reporting COVID-19 outcomes with mRNA vaccines in older adults aged ≥ 50 years by first cross-checking relevant published SLRs. Based on the cutoff date from a previous similar SLR, we then searched the WHO COVID-19 Research Database for relevant articles published between April 9, 2022, and June 2, 2023. Outcomes of interest were SARS-CoV-2 infection, symptomatic SARS-CoV-2 infection, severe SARS-CoV-2 infection, COVID-19–related hospitalization, and COVID-19–related death following ≥ 2 vaccine doses. Random effects meta-analysis models were used to pool risk ratios (RRs) across studies. Heterogeneity was evaluated using chi-square testing. Evidence certainty was assessed per GRADE framework.
Results
Twenty-four non-randomized real-world studies reporting clinical outcomes with mRNA vaccines in individuals aged ≥ 50 years were included in the meta-analysis. Vaccination with mRNA-1273 was associated with significantly lower risk of SARS-CoV-2 infection (RR 0.72 [95% confidence interval (CI) 0.64‒0.80]), symptomatic SARS-CoV-2 infection (RR 0.72 [95% CI 0.62‒0.83]), severe SARS-CoV-2 infection (RR 0.67 [95% CI 0.57‒0.78]), and COVID-19–related hospitalization (RR 0.65 [95% CI 0.53‒0.79]) but not COVID-19–related death (RR 0.80 [95% CI 0.64‒1.00]) compared with BNT162b2. There was considerable heterogeneity between studies for all outcomes (I2 > 75%) except death (I2 = 0%). Multiple subgroup and sensitivity analyses excluding specific studies generally demonstrated consistent results. Certainty of evidence across outcomes was rated as low (type 3) or very low (type 4), reflecting the lack of randomized controlled trial data.
Conclusion
Meta-analysis of 24 observational studies demonstrated significantly lower risk of asymptomatic, symptomatic, and severe infections and hospitalizations with the mRNA-1273 versus BNT162b2 vaccine in older adults aged ≥ 50 years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out the study? |
The coronavirus disease 2019 (COVID-19) pandemic has disproportionately affected older adults, as this population is generally more susceptible to infection and severe outcomes because of immune senescence and underlying comorbidities. |
The two available mRNA vaccines mRNA-1273 and BNT162b2 have demonstrated high efficacy against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in phase 3 clinical trials, including among older adults. |
What was learned from the study? |
To inform COVID-19 vaccine selection, this systematic literature review and meta-analysis assessed the comparative effectiveness of mRNA-1273 versus BNT162b2 among older adults in real-world settings. |
Vaccination with homologous primary or booster mRNA-1273 was associated with significantly lower risk of infection (including asymptomatic, symptomatic, and severe infections) and hospitalization due to COVID-19 than vaccination with BNT162b2 in older adults aged ≥ 50 years. |
Introduction
As of October 2023, the global coronavirus disease 2019 (COVID-19) pandemic has resulted in > 771.4 million reported infections and > 6.9 million deaths due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. COVID-19 has disproportionately affected older adults [2,3,4,5]. Worldwide, older adults aged ≥ 60 years accounted for 80% of COVID-19–associated deaths reported to the World Health Organization (WHO) via detailed weekly surveillance from January 2020 to December 2021 and were estimated to account for 82% of deaths based on the WHO excess mortality model [4]. Immune senescence and underlying comorbidities make older adults generally more susceptible to COVID-19 and associated severe outcomes. Several studies have identified older age as a primary risk factor for severe illness with COVID-19 [6,7,8], with one study demonstrating similar performance between a risk score that was based on age alone versus a validated risk score incorporating the effects of multiple underlying comorbidities (POINTED score) [9]. Importantly, the WHO has identified older adults (commonly defined by age cutoffs of 50–60 years, depending on the country) as a high-priority group for COVID-19 vaccination [10], and many countries have prioritized vaccination of the older population [9].
A previous meta-analysis of 32 studies in older adults aged ≥ 55 years found that vaccination with either one of the two vaccines employing novel messenger ribonucleic acid (mRNA) technology provided the highest protection against COVID-19 compared with other vaccine types [11]. The mRNA vaccines were developed and granted emergency use authorization in late 2020 to globally mitigate the spread of SARS-CoV-2: mRNA-1273 (Spikevax®; Moderna, Inc., Cambridge, MA, USA) [12] and BNT162b2 (Comirnaty®; Pfizer/BioNTech, New York, NY, USA/Mainz, Germany) [13]. Phase 3 trials of these vaccines demonstrated high vaccine efficacy against SARS-CoV-2 infection when administered as two-dose regimens (94.1% and 95.0% effectiveness with mRNA-1273 and BNT162b2, respectively) [14, 15], with subgroup analyses also confirming high vaccine efficacy in older participants (aged ≥ 65 years) [14, 15].
Although both mRNA-1273 and BNT162b2 are based on mRNA technology, their formulations differ. For example, the mRNA-1273 vaccine contains more active ingredient (100 µg of mRNA for primary; 50 µg for booster) than the BNT162b2 vaccine (30 µg of mRNA for both primary and booster) [12, 13, 16, 17] and uses a different lipid nanoparticle delivery system [18,19,20]. As shown with other respiratory vaccines [21, 22], and as demonstrated in immunocompromised individuals [23], these differences may impact vaccine effectiveness in older adults.
Data comparing the effectiveness of COVID-19 vaccines are needed to inform vaccine selection and to support healthcare policy and reimbursement decision-making at the population level [24,25,26,27,28]. Such comparative effectiveness data can help inform procurement decisions to ensure that healthcare providers and their patients have access to the most effective vaccines. However, there have been no head-to-head comparisons of the mRNA-1273 and BNT162b2 vaccines in randomized controlled trials (RCTs). Thus, there remains a need to synthesize evidence across real-world studies to provide robust information about the comparative effectiveness of the two mRNA vaccines, particularly in high-risk populations, such as older adults.
To compare the effectiveness of mRNA-1273 versus BNT162b2 against SARS-CoV-2 infections and COVID-19 outcomes (severe infections, hospitalizations, and deaths) in older adults, we performed a systematic literature review and pairwise meta-analysis of previously published studies. Our analysis followed the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework [29] used by national immunization advisory groups when developing recommendations [30]. Specifically, our research aimed to address the following question: ‘Is mRNA-1273 more effective than BNT162b2 at preventing SARS-CoV-2 infections and COVID-19–related hospitalizations and deaths in older adults aged ≥ 50 years?’
Methods
Search Strategy and Study Selection
This systematic literature review and meta-analysis is registered in PROSPERO(CRD42023443149) and was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 framework [31]. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Studies were identified using a two-step search procedure. First, the WHO COVID-19 Research Database was searched to identify systematic literature reviews on COVID-19 vaccination outcomes in the general population published between March 2020 and 19 April 2023. Sixteen of 67 systematic reviews identified were relevant (Supplementary Material Table S1) and were cross-checked for articles to be included in full-text assessment of whether additional criteria for our analysis were met, as described below. One prior systematic review identified had similar objectives to the current study [11], and all studies included in this prior review were included for full-text assessment. A total of 243 studies for full-text screening were identified from this first step. The main search was then conducted in the WHO COVID-19 Research Database to identify relevant studies published since the prior similar systematic review [11], which included studies from database inception through 9 April 2022 to 2 June 2023. Notably, although the WHO COVID-19 Research Database remains searchable, updates ceased in June 2023 [32]; thus, content spans March 2020 through June 2023. Databases searched include MEDLINE/PubMed, International Clinical Trials Registry Platform, Embase, EuropePMC, medRxiv, Web of Science, ProQuest Central, Academic Search Complete, Scopus, and COVIDWHO. The main database search identified an additional 1012 studies for full-text screening. The main search strategy is summarized in Supplementary Material Table S2.
RCTs, observational studies, and any real-world evidence published as full-text manuscripts, letters, commentaries, abstracts, or posters were included if they reported prespecified COVID-19 outcomes (described below) in older adults aged ≥ 50 years who received mRNA-1273 or BNT162b2 within the same study (studies with ≤ 10% of the study population aged ≤ 50 years included). Studies could include participants who had comorbidities and those who were immunocompetent or defined as clinically extremely vulnerable (CEV) with conditions in CEV group 3, as categorized by Canadian Health Services [33] (studies with ≤ 10% of participants with CEV group 1 and 2 conditions were included). Diabetes was considered a CEV group 3 condition regardless of whether the patient was being treated with insulin. Only studies reporting the outcomes of interest for participants who received ≥ 2 vaccine doses were included, with a preference for three-dose data where available. If a study did not report the outcomes for participants who received three doses, then two- or four-dose data were considered. Only homologous dose series (≥ 2 doses of mRNA-1273 or ≥ 2 doses BNT162b2) were included in analysis.
Outcomes of interest were vaccine efficacy or effectiveness against SARS-CoV-2 infection (defined as asymptomatic or symptomatic infection with positive test or a COVID-19 diagnosis code [U07.1]), laboratory-confirmed symptomatic SARS-CoV-2 infection (defined as positive test with symptoms including but not limited to fever, cough, shortness of breath, and sudden onset of anosmia/ageusia; in some countries, runny nose was also included in the case definition), severe SARS-CoV-2 infection (defined specifically as severe infection or as hospitalization or death, as reported in the study; primarily defined by severe infection, followed by hospitalization and lastly by death if data on multiple endpoints were available), COVID-19–related hospitalization (defined as intubation, hospitalization, or admission to intensive care unit with positive test for SARS-CoV-2 infection within 5 days before to 28 days from admission; cases with information on intubation but not hospitalization were assumed to be hospitalized), or COVID-19–related death (defined as deaths occurring after a positive test for SARS-CoV-2 infection without previously declared recovery or another clear cause of death reported). A positive SARS-CoV-2 test could be based on any of the following methods, as reported by individual studies: reverse transcription polymerase chain reaction (PCR), rapid antigen test, or dried blood spot seropositivity for anti-nucleocapsid immunoglobulin G antibodies by validated enzyme-linked immunosorbent assay. Infections were considered if they occurred ≥ 7 days after the last vaccination. Only those studies that reported the following data were included in the meta-analysis: number of events and sample size per arm, or vaccine effectiveness (VE) per arm and subgroup derived as 1–risk ratio (RR), 1–odds ratio (OR), 1–hazard ratio (HR), or 1–incidence rate ratio (IRR). For the analyses of VE, if only VE data and total numbers of participants by vaccine arm were available, then the weighted average VE for all age groups among individuals aged ≥ 50 years was computed. Weighted average was calculated as the sum of the VE in all age groups in a vaccine arm divided by the total number of participants in that arm. If only VE data were available without participant numbers by vaccine arm, then VE in the age group that most closely matched the data within the studies in the meta-analysis was selected.
Studies in pregnant women, current or former smokers, and physically inactive participants; studies including only immunocompromised individuals with conditions within CEV groups 1 and 2; and studies with only safety and/or immunogenicity outcomes were excluded. The population, exposure, comparison, and outcomes used in the systematic literature review are summarized in Supplementary Material Table S3. Two independent reviewers selected studies using a two-level approach; discrepancies were resolved by consensus or by a third reviewer. In level 1, titles and abstracts were screened against inclusion criteria; then, in level 2, articles not excluded at level 1 underwent full-text screening against the selection criteria.
Data Extraction and Quality Assessment
Study design details, baseline characteristics of study participants, vaccine received and dosing details, and vaccine efficacy/effectiveness outcomes were extracted from the selected studies. Risk of bias was assessed using the Newcastle-Ottawa Scale [34] for observational studies. The certainty of evidence was evaluated based on GRADE criteria [29, 30].
Statistical Analysis
Random effects meta-analysis models were used to pool RRs and to estimate absolute effects as risk difference (RD) per 100,000 individuals across the included studies, comparing mRNA-1273 to BNT162b2. The inverse variance method was applied for the random effects models [35]. Details regarding methodology of the analyses are included in the Supplementary Material (Appendix 1). Briefly, a standard pairwise meta-analysis was conducted using RRs instead of number of events and sample size per arm as the data input. However, due to differences in how outcomes were reported across studies, a conversion approach [36,37,38] was implemented. For studies that reported the number of events and sample size per arm, unadjusted RRs were estimated straightforwardly. For studies that exclusively reported VE, instead of number of events and sample size per arm, RR was estimated either as “1–VE” (for studies reporting VE as 1–RR) or from VE through optimal approximate conversions of contrast-based data (Supplementary Material Figure S1). As a sensitivity analysis, a second-order meta-analysis approach was implemented to avoid the assumptions based on converting contrast-based data in the conversion approach. With this approach, data from studies reporting number of events and sample size were pooled in one meta-analysis, and data from studies reporting only VE were pooled in a second meta-analysis (i.e., without distinction as to how VE was estimated and without any conversion). In the second-order meta-analysis, the pooled results from these separate meta-analyses on RRs informed the analysis, resulting in the final RR estimate. Absolute effects (RD) cannot be reliably estimated using this second-order approach, so this method was used only for analysis of RR.
As additional sensitivity analyses, outcomes were assessed in the following subgroups: individuals aged ≥ 65 years; individuals aged ≥ 75 years; individuals who received exclusively three doses of the same vaccine; individuals aged ≥ 50 years, excluding those with disease conditions categorized in CEV groups 1 or 2; individuals infected with the SARS-CoV-2 Delta variant (i.e., dominant variant during study time period); and excluding those studies that reported only VE.
Publication bias was assessed by visual examination of funnel plots and Egger’s regression test for asymmetry [39, 40]. Heterogeneity across studies was evaluated using chi-square testing [41], with the percentage of variation across studies estimated using the I2 statistic (scale of 0–100%, with 0% meaning no evidence of heterogeneity; see Supplementary Material Appendix 1). Results were summarized in forest plots to display the effect estimates with 95% confidence intervals (CIs) for the individual studies and the pooled estimate of the meta-analysis. The meta-analyses were conducted in R (v4.3.1), using the meta [42] and metafor [43] packages.
Results
Search Results and Included Studies
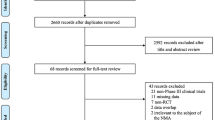
In total, 1255 abstracts were identified from either the 16 relevant SLRs that were cross-checked (n = 243) or the main search in the WHO COVID-19 database (n = 1012) and screened for inclusion (Fig. 1). Of these, 25 studies (all non-randomized) reported results for the clinical outcomes of interest in individuals aged ≥ 50 years, 24 of which were included in the meta-analysis (one study [44] was excluded because it reported only RR and thus did not meet the prespecified criterion of reporting number of events and sample size or VE).
PRISMA flow diagram. aDatabases searched include ICTRP, EMBASE, EuropePMC, medRxiv, Web of Science, ProQuest Central, Academic Search Complete, Scopus, and COVIDWHO. bSixteen recently published SLRs and internal documents from Moderna, Inc., were cross-checked. cOne study [44] was excluded from the network meta-analysis because the presented data were not comparable to the data from other studies. SLR systematic literature review, WHO World Health Organization
Characteristics of each of the studies included in the meta-analysis are summarized in Table 1. Of the 24 studies, 1 was industry-sponsored. Overall, the studies included > 3.9 million older adults (aged ≥ 50 years) vaccinated with mRNA-1273 and > 5.2 million vaccinated with BNT162b2. Most studies involved North American (Canada, n = 2 [45, 46]; USA, n = 11 [47,48,49,50,51,52,53,54,55,56,57]) or European (Belgium, n = 1 [58]; Greece, n = 1 [59]; Hungary, n = 2 [60, 61]; Norway, n = 1 [62]; Netherlands, n = 1 [63]; Spain, n = 2 [64, 65]; multiple countries, n = 1 [66]) populations. Although most studies included general population samples, two were restricted to nursing home or retirement home residents [45, 48] and two were restricted to Veteran’s Affairs populations in the US [47, 49]. Most studies specified the Delta variant as the SARS-CoV-2 variant of concern [47, 48, 51,52,53, 55, 56, 58, 59, 61,62,63, 66]; 5 studies specified the Alpha variant [51, 54, 58,59,60] and 4 specified the Omicron variant [45, 46, 55, 58]. Some studies with longer follow-up periods collected data during multiple COVID-19 seasons and therefore reported data on multiple variants, either not further specified or in separate subgroups. We conducted a subgroup analysis in patients infected with the Delta variant because of the large number of available studies; subgroup analysis for other variants was deemed unfeasible because of sparse data. In the majority of studies, positivity for SARS-CoV-2 infection was determined using PCR or an antigen test; however, four studies did not specify the testing method [49, 50, 52, 56] and one study used a nasopharyngeal PCR test and/or circulating antinucleocapsid IgG antibodies [45].
Based on the risk of bias assessment for non-randomized studies, most of the studies included in the meta-analysis had no serious risk of bias; however, there was serious risk of bias in four studies [45, 50, 59, 64], and risk of bias was not estimable for one study [55] (Supplementary Material Table S4).
SARS-CoV-2 Infection
In meta-analysis of 22 studies reporting the outcome of SARS-CoV-2 infection in older adults aged ≥ 50 years, vaccination with mRNA-1273 was associated with significantly lower risk of SARS-CoV-2 infection compared with vaccination with BNT162b2 (RR 0.72 [95% CI 0.64–0.80]; Table 2 and Figs. 2 and 3). The RD was estimated as 442 fewer (95% CI 570 fewer to 313 fewer) SARS-CoV-2 infections per 100,000 people vaccinated. There was considerable heterogeneity between the studies (RR I2 = 94.4%; RD I2 = 98.4%). The certainty of evidence was graded as type 4 (very low) because of imprecision and indirectness resulting from the varying outcome definitions used for infection and inclusion of non-randomized studies (Table 2). In a sensitivity analysis using the second-order methodological approach, vaccination with mRNA-1273 was associated with significantly fewer SARS-CoV-2 infections compared with BNT162b2 (RR 0.72 [95% CI 0.62–0.85]; I2 = 0%), consistent with the base case analysis (Fig. 4A and Supplementary Material Figure S2A).
Meta-analysis results comparing the mRNA-1273 versus BNT162b2 COVID-19 vaccines in the overall population of older adults aged ≥ 50 years by study for A SARS-CoV-2 infection; B laboratory-confirmed symptomatic SARS-CoV-2 infection; C severe SARS-CoV-2 infection; D hospitalization due to COVID-19; and E death due to COVID-19. CI confidence interval, COVID-19 coronavirus disease 2019, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2
Summary of sensitivity meta-analyses on clinical effectiveness outcomes of the mRNA-1273 versus BNT162b2 COVID-19 vaccines A using the second-order methodological approacha and in subgroups of B older adults aged ≥ 65 years and C older adults aged ≥ 50 years who received exclusively three doses. aResults of the second-order methodological approach for the outcome of symptomatic infection are not presented because the results are identical to the results of the main analysis (no conversion was necessary for this outcome in the main analysis). CI confidence interval, COVID-19 coronavirus disease 2019, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2
In a subgroup analysis of ten studies reporting the outcome of SARS-CoV-2 infection in adults aged ≥ 65 years, mRNA-1273 vaccination was also associated with significantly fewer infections compared with BNT162b2 vaccination (RR 0.74 [95% CI 0.62–0.88]; RD 216 fewer cases per 100,000 vaccinated [95% CI 333 fewer to 100 fewer]; Table 3, Fig. 4B and Supplementary Material Figure S3A). Subgroup analysis of seven studies reporting this outcome in individuals aged ≥ 50 years who received exclusively three vaccine doses also found that mRNA-1273 was associated with fewer infections versus BNT162b2 (RR 0.64 [95% CI 0.54–0.74]; RD 1098 fewer cases per 100,000 vaccinated [95% CI 1535 fewer to 661 fewer]; Table 3, Fig. 4C and Supplementary Material Figure S4A). As in the overall population analysis, the certainty of evidence in these two subgroups was graded as type 4 (very low) because of imprecision and varying outcome definitions (Table 3), and there was considerable heterogeneity between studies (RR I2 = 89.5% for the ≥ 65 years of age subgroup; I2 = 80.8% for the 3-dose subgroup). Additional subgroup analyses in older adults aged ≥ 75 years; in older adults aged ≥ 50 years, excluding individuals with CEV group 1 and 2 conditions; in older adults aged ≥ 50 years infected with the Delta variant; and excluding those studies that included only VE data were generally consistent with the findings from the overall meta-analysis (Supplementary Material Figures S5A, S6A, S7A, and S8A).
Laboratory-Confirmed Symptomatic SARS-CoV-2 Infection
Five studies were included in the meta-analysis of laboratory-confirmed symptomatic SARS-CoV-2 infection in individuals aged ≥ 50 years (Table 2). Vaccination with mRNA-1273 was associated with significantly fewer SARS-CoV-2 symptomatic infections versus vaccination with BNT162b2 (RR 0.72 [95% CI 0.62–0.83]; Figs. 2 and 3). The RD was estimated as 609 fewer symptomatic infections per 100,000 individuals vaccinated (95% CI 980 fewer to 238 fewer cases). Heterogeneity between studies was also considerable for this outcome (RR I2 = 75.1%; RD I2 = 96.2%). The certainty of evidence was graded as type 3 (low) due to imprecision, with a lower grading assigned due to inclusion of non-randomized studies (Table 2). Possible publication bias was noted for this outcome based on Egger’s regression test (P < 0.05) (Supplementary Material Figure S9B). Because no VE data were used in the base case meta-analysis of symptomatic SARS-CoV-2 infections, no conversion was necessary. Therefore, results from the second-order methodological approach were identical to the base case results presented in Figs. 2 and 3.
Subgroup analysis based on two studies in individuals aged ≥ 65 years also found significantly reduced risk of symptomatic SARS-CoV-2 infections with mRNA-1273 versus BNT162b2 vaccination (RR 0.74 [95% CI 0.56–0.97]; RD 3030 fewer cases per 100,000 vaccinated [95% CI 8844 fewer to 2784 more cases]; Table 3, Fig. 4B and Supplementary Material Figure S3B). Similarly, in meta-analysis of three studies that included individuals aged ≥ 50 years who received exclusively three doses of vaccine, mRNA-1273 was associated with lower risk of symptomatic infections compared with BNT162b2 (RR 0.74 [95% CI 0.61–0.90]; RD 114 fewer cases per 100,000 individuals vaccinated [95% CI 338 fewer to 111 more]; Table 3, Fig. 4C, Supplementary Material Figure S4B). As in the overall meta-analysis, heterogeneity between studies was considerable for these subgroups (RR I2 = 90.8% and 79.0%, respectively). The certainty of evidence was graded as type 4 (very low) for both subgroups (Table 3). Results of additional subgroup analyses in adults aged ≥ 75 years; adults aged ≥ 50 years, excluding individuals with CEV group 1 and 2 conditions; and adults aged ≥ 50 years infected with the Delta variant were generally consistent with the findings from the overall meta-analysis (Supplementary Material Figures S5B, S6B, and S7B). There were no studies evaluating the outcome of laboratory-confirmed symptomatic SARS-CoV-2 infection that exclusively reported VE data.
Severe SARS-CoV-2 Infection
Based on meta-analysis of 12 studies, vaccination with mRNA-1273 was associated with significantly fewer severe SARS-CoV-2 infections compared with vaccination with BNT162b2 (RR 0.67 [95% CI 0.57–0.78]; Table 2 and Figs. 2 and 3). This result corresponds to an estimated RD of 20 fewer severe infections per 100,000 individuals vaccinated with mRNA-1273 versus BNT162b2 (95% CI 29 fewer to 11 fewer cases). There was considerable heterogeneity across studies for this outcome (RR I2 = 78.1%; RD I2 = 86.0%). Evidence certainty was graded as type 4 (very low) because of imprecision and varying definitions used for severe infection (defined as severe infection, or hospitalization, or death; Table 2). Possible publication bias was noted for this outcome based on Egger’s regression test (P < 0.05; Supplementary Material Figure S9C). Consistent with the findings from the base case analysis, sensitivity analysis using the second-order methodological approach also found that vaccination with mRNA-1273 was associated with significantly fewer severe SARS-CoV-2 infections compared with vaccination with BNT162b2 in older adults aged ≥ 50 years (RR 0.66 [95% CI 0.59–0.75]; Fig. 4A and Supplementary Material Figure S2B).
In subgroup analyses, mRNA-1273 was associated with significantly fewer severe SARS-CoV-2 infections compared with vaccination with BNT162b2 in older adults aged ≥ 65 years (eight studies; RR 0.65 [95% CI 0.51–0.83]; RD 24 fewer severe infections per 100,000 individuals vaccinated [95% CI 41 fewer to 7 fewer]) and in adults aged ≥ 50 years who received exclusively three vaccine doses (four studies; RR 0.62 [95% CI 0.44–0.88]; RD 10 fewer severe infections per 100,000 individuals vaccinated [95% CI 16 fewer to 3 fewer]; Table 3, Fig. 4B and C, and Supplementary Material Figure S3C and S4C). There was substantial heterogeneity across studies for both subgroups (RR I2 = 63.5% and 60.4%, respectively). Evidence certainty was graded as type 4 (very low; Table 3). Similar to the findings from the overall meta-analysis, mRNA-1273 was associated with reduced risk of severe SARS-CoV-2 infection in additional subgroup analyses of individuals ≥ 75 years of age, individuals (aged ≥ 50 years) without CEV group 1 or 2 conditions, individuals (aged ≥ 50 years) infected with the Delta variant, and in the subgroup excluding those studies that included only VE data (Supplementary Material Figures S5C, S6C, S7C, and S8B).
Hospitalization Due to COVID-19
Based on a meta-analysis of eight studies, vaccination with mRNA-1273 was associated with significantly lower risk of hospitalization due to COVID-19 in individuals aged ≥ 50 years compared with vaccination with BNT162b2 (RR 0.65 [95% CI 0.53–0.79]; Table 2 and Figs. 2 and 3). The estimated RD was 23 fewer COVID-19 hospitalizations per 100,000 individuals vaccinated (95% CI 34 fewer to 12 fewer). Heterogeneity across studies was considerable (RR I2 = 85.4%; RD I2 = 90.3%). The certainty of evidence grade was type 3 (low) for this outcome due to imprecision and inclusion of non-randomized studies (Table 2). The sensitivity analysis using the second-order methodological approach found that vaccination with mRNA-1273 was associated with significantly fewer COVID-19–related hospitalizations compared with vaccination with BNT162b2 (RR 0.63 [95% CI 0.57–0.70]), consistent with the base case analysis (Fig. 4A and Supplementary Material Figure S2C).
Based on seven studies of COVID-19–related hospitalization in the subgroup of older adults aged ≥ 65 years, vaccination with mRNA-1273 was associated with significantly reduced risk of hospitalization compared with vaccination with BNT162b2 (RR 0.69 [95% CI 0.53–0.89]; RD 82 fewer hospitalizations per 100,000 individuals vaccinated [95% CI 134 fewer to 29 fewer]; Table 3 and Fig. 4B and Supplementary Material Figure S3D). As in the overall meta-analysis, there was considerable heterogeneity across studies (RR I2 = 72.0%), and the evidence certainty was graded as type 3 (low; Table 3). Vaccination with mRNA-1273 was also associated with significantly reduced risk of hospitalization compared with vaccination with BNT162b2 among individuals aged ≥ 50 years who received three vaccine doses based on meta-analysis of three studies (RR 0.55 [95% CI 0.37–0.82]; RD 11 fewer hospitalizations per 100,000 individuals vaccinated [95% CI 18 fewer to 3 fewer]; Table 3 and Fig. 4C and Supplementary Material Figure S4D). There was moderate heterogeneity across studies (RR I2 = 47.5%), and the evidence certainty was graded as type 4 (very low; Table 3). Additional subgroup analyses in adults aged ≥ 75 years; adults aged ≥ 50 years, excluding individuals with CEV group 1 and 2 conditions; adults aged ≥ 50 years infected with the Delta variant; and excluding those studies that included only VE data were generally consistent with the findings from the overall meta-analysis (Supplementary Material Figures S5D, S6D, S7D, and S8C).
Death Due to COVID-19
In meta-analysis of seven studies reporting mortality in individuals aged ≥ 50 years, vaccination with mRNA-1273 was associated with a numerically lower but not significantly lower risk of COVID-19–related death compared with vaccination with BNT162b2 (RR 0.80 [95% CI 0.64–1.00]). The estimated RD was 2 fewer deaths per 100,000 people vaccinated (95% CI 6 fewer to 2 more) (Table 2 and Figs. 2 and 3). No evidence of heterogeneity between the studies was observed in the RR analysis (I2 = 0%), although heterogeneity was moderate for the estimation of RD (I2 = 48.8%). The certainty of evidence was graded as type 3 (low) for this outcome because of imprecision and inclusion of non-randomized studies (Table 2). In the sensitivity analysis using the second-order approach, mRNA-1273 vaccination was associated with numerically reduced risk of death due to COVID-19 compared with BNT162b2 vaccination, but this was also not statistically significant (RR 0.77 [95% CI 0.59–1.01]; Fig. 4A and Supplementary Material Figure S2D).
In subgroup analysis of four studies reporting this outcome in older adults aged ≥ 65 years, vaccination with mRNA-1273 was associated with fewer COVID-19 deaths versus vaccination with BNT162b2 (RR 0.72 [95% CI 0.54–0.98]; RD 11 fewer deaths per 100,000 individuals vaccinated [95% CI 19 fewer to 4 fewer]) (Table 3, Fig. 4B, and Supplementary Material Figure S3E). The evidence suggested that the heterogeneity across studies might not be important (RR I2 = 10.9%). The evidence certainty was graded as type 4 (very low) in this analysis because of imprecision and limited evidence (Table 3). There was no statistically significant difference between the mRNA vaccines against the outcome of COVID-19–related deaths in the subgroup of individuals aged ≥ 50 years who received exclusively three vaccine doses, based on analysis of two studies (RR 1.01 [95% CI 0.64–1.57]; Table 3, Fig. 4C, and Supplementary Material Figure S4E). The mRNA-1273 vaccine was associated with reduced risk of COVID-19–related death compared with BNT162b2 when individuals with CEV1/2 group conditions were excluded (Supplementary Material Figure S6E). There was no statistically significant difference in mortality risk between mRNA vaccines in subgroup analyses of individuals aged ≥ 75 years, individuals aged ≥ 50 years exposed to Delta variant, or in the subgroup excluding those studies with only VE data (Supplementary Material Figures S5E, S7E, and S8D).
Discussion
This meta-analysis of 24 studies in older adults aged ≥ 50 years found that vaccination with mRNA-1273 was statistically significantly associated with lower risk of SARS-CoV-2 infections, including asymptomatic, symptomatic, and severe infections, as well as hospitalizations due to COVID-19 compared with vaccination with BNT162b2. To our knowledge, this is the first such analysis of pairwise real-world evidence in adults aged 50 years or older. This evidence helps inform considerations about which vaccine to choose for older adults, and also helps inform healthcare policy decision-making. In particular, comparative effectiveness data are important to consider in reimbursement and procurement decisions to ensure that healthcare providers and their patients have access to the most effective vaccines [24,25,26,27,28].
Older age has consistently been identified as a primary risk factor for worse outcomes with COVID-19 [6,7,8], with older adults accounting for the majority of COVID-19–related deaths [2, 3, 5, 67]. This meta-analysis provides evidence for improved outcomes with the mRNA-1273 vaccine compared with the BNT162b2 vaccine in older adults. Similarly, high-dose and adjuvanted influenza vaccines have demonstrated improved outcomes over standard dose influenza vaccines in older adults [21, 22]; as a result, these vaccines are preferentially recommended for the elderly population in many countries [68, 69]. Immunology studies have also reported higher antibody production with the mRNA-1273 vaccine compared with the BNT162b2 vaccine [19].
Findings from the sensitivity analysis using the second-order meta-analysis approach were consistent with the overall results among older adults aged ≥ 50 years. Consistent findings were also observed in subgroup analyses among adults aged ≥ 65 years or ≥ 75 years, in adults aged ≥ 50 years who received exclusively three doses, in those who did not have CEV groups 1 and 2 conditions, and those infected by the Delta variant, and in the subgroup excluding studies that reported only VE. Across these subgroups, vaccination with mRNA-1273 was associated with significantly fewer infections, symptomatic infections, and severe infections compared with vaccination with BNT162b2. Vaccination with mRNA-1273 was also associated with significantly fewer hospitalizations compared with vaccination with BNT162b2 in each subgroup. For the outcome of COVID-19–related death, there was no significant difference observed between the vaccines in any of the subgroups, except among those who did not have CEV groups 1 and 2 conditions, where vaccination with mRNA-1273 was associated with significantly fewer deaths compared with vaccination with BNT162b2. Overall, the findings from the base-case analysis were confirmed by a broad range of sensitivity analyses considering different subgroups as well as different methodologies (i.e., second-order approach and exclusion of VE studies), suggesting that the findings of the meta-analysis are robust.
Limitations of this systematic review and meta-analysis should be considered. Because all the studies included in the analysis were observational in nature, the certainty of evidence was graded as low or very low (type 3 or below). Furthermore, four of the 24 studies included in the meta-analysis had a serious risk of bias, and risk of bias was not estimable for one additional study due to lack of sufficient information. Importantly, the tight timelines for developing variant-adapted vaccines for COVID-19 limits the feasibility of large RCTs. In this context, estimates of the comparative effectiveness of COVID-19 vaccines based on real-world evidence provides crucial information to address important clinical questions and inform policy decisions regarding vaccination [70]. Nevertheless, higher quality real-world evidence studies on vaccine effectiveness are needed, particularly for the outcomes of COVID-19–related hospitalizations and deaths. Possible publication bias was noted based on Egger’s regression test (P < 0.05) for the outcomes of symptomatic infections and severe infections. However, the number of studies reporting symptomatic infections was small (n = 5), limiting the power of Egger’s regression test to accurately distinguish chance from true asymmetry. A combination of various endpoints, including severe infection (as defined by the study), hospitalization, and death, was used to define a composite severe infections outcome in this meta-analysis, introducing additional heterogeneity. This may have contributed to the significant asymmetry observed for this outcome. Publication bias was not detected based on Egger’s test for outcomes with sufficient numbers of studies in the evidence base (i.e., for SARS-2-CoV-2 infections, hospitalizations, and deaths). The studies included in our meta-analysis showed a large amount of heterogeneity. This finding is possibly a reflection of the complex interactions between vaccination and contextual factors as they operate in the real world. However, such heterogeneity does introduce challenges in predicting true vaccine effectiveness under a given regimen, or for a given population. Various factors could have driven the observed heterogeneity, including differences in study populations, statistical approaches employed, definitions of outcomes (e.g., for severe COVID-19), analyzed time points after vaccination, and vaccination schedules and regimens. Such high heterogeneity may also be expected in older populations, in part due to the large heterogeneity in health status associated with underlying comorbidities, for example. Meta-regression accounting for some of the factors plausibly driving heterogeneity (such as varying time points of analysis after vaccination and vaccination schedules and regimes) could not be conducted because of sparse data. However, we performed multiple subgroup analyses to account for age differences (i.e., restricted to individuals aged ≥ 65 years and ≥ 75 years), differences in number of vaccine doses (i.e., restricted to individuals who received three doses), underlying medical conditions (i.e., excluding those with CEV group 1 and 2 conditions), and SARS-CoV-2 variant (i.e., restricted to a single variant of concern [Delta]) to better understand the source of heterogeneity. Heterogeneity continued to be observed across these sub-analyses. Notably, high heterogeneity has also been noted in meta-analyses of influenza vaccine effectiveness in older adults [21, 69, 71]. Future studies and reviews examining which factors predict when, where, and for whom the vaccines show differential effectiveness would be beneficial to address possible disparities in protection. Despite the high heterogeneity we observed, comprehensive sensitivity analyses considering only subsets of studies (i.e., excluding studies or groups of studies) were conducted, results of which highlight the robustness of effect sizes and the conclusion of the overall meta-analysis [72].
Our evidence synthesis has several considerable strengths. First, we used broad search terms and high-quality systematic literature review methodology, which included training reviewers and validating the included studies and extracted data. Second, we used advanced meta-analytical methods to include both studies reporting event and participant numbers by vaccine arm as well as studies reporting only VE. This approach allowed for inclusion of all available data, considering both within- and between-study variability, resulting in more robust and reliable conclusions than would be possible if either only binary data or only VE data were included. We also carried out a sensitivity analysis using a second-order meta-analytical model which demonstrated similar results to the main analysis, corroborating the robustness of the data and the analytical methods employed. Finally, this evidence synthesis and meta-analysis provides important updates compared with previous analyses, notably providing results on the comparative effectiveness of the two available mRNA vaccines in preventing SARS-CoV-2 infections and associated severe outcomes.
Conclusions
Vaccination with mRNA-1273 was associated with significantly fewer asymptomatic, symptomatic, and severe infections and hospitalizations due to COVID-19 than vaccination with BNT162b2 in older adults aged ≥ 50 years, and these differences generally persisted among subgroups of patients, including among older adults aged ≥ 65 years and adults aged ≥ 50 years who received three doses of the same vaccine. By providing synthesized data on the comparative effectiveness of the two available COVID-19 mRNA vaccines, these results can assist healthcare policy decision-makers who wish to optimize vaccination programs at the population level as well as healthcare professionals making individual-based recommendations to their patients.
Data Availability
All original data generated or analyzed during this study are included in this published article/as Supplementary Material files. Further inquiries can be directed to the corresponding author.
References
World Health Organization (WHO). WHO coronavirus (COVID-19) dashboard 2023 [updated Oct 18, 2023]. https://covid19.who.int/.
Ahmad FB, Cisewski JA, Xu J, Anderson RN. COVID-19 mortality update—United States, 2022. MMWR Morb Mortal Wkly Rep. 2023;72(18):493–6.
World Health Organization (WHO). WHO coronavirus (COVID-19) dashboard: confirmed and probable COVID-19 cases and deaths by age 2023 [updated October 16, 2023]. https://data.who.int/dashboards/covid19/cases?n=c.
Wong MK, Brooks DJ, Ikejezie J, Gacic-Dobo M, Dumolard L, Nedelec Y, et al. COVID-19 mortality and progress toward vaccinating older adults—World Health Organization, Worldwide, 2020–2022. MMWR Morb Mortal Wkly Rep. 2023;72(5):113–8.
Tejada-Vera B, Kramarow EA. COVID-19 mortality in adults aged 65 and over: United States, 2020. NCHS Data Brief. 2022;446:1–8.
Hu L, Chen S, Fu Y, Gao Z, Long H, Ren HW, et al. Risk factors associated with clinical outcomes in 323 coronavirus disease 2019 (COVID-19) hospitalized patients in Wuhan, China. Clin Infect Dis. 2020;71(16):2089–98.
Petrilli CM, Jones SA, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966.
Sung J, Choudry N, Bachour R. Development and validation of a simple risk score for diagnosing COVID-19 in the emergency room. Epidemiol Infect. 2020;148:e273.
Jacob J, Tesch F, Wende D, Batram M, Loser F, Weidinger O, et al. Development of a risk score to identify patients at high risk for a severe course of COVID-19. Z Gesundh Wiss. 2023:1–10. https://doi.org/10.1007/s10389-023-01884-7.
World Health Organization (WHO). WHO SAGE roadmap on uses of COVID-19 vaccines in the context of OMICRON and substantial population immunity 2023 [updated March 30, 2023]. https://www.who.int/publications/i/item/WHO-2019-nCoV-Vaccines-SAGE-Roadmap.
Li Z, Liu S, Li F, Li Y, Li Y, Peng P, et al. Efficacy, immunogenicity and safety of COVID-19 vaccines in older adults: a systematic review and meta-analysis. Front Immunol. 2022;13:965971.
SPIKEVAX. mRNA-1273. Cambridge: ModernaTX, Inc.; 2022.
COMIRNATY. BNT162b2. New York: Pfizer/BioNTech; 2022.
Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–16.
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–15.
Moderna I. Fact sheet for healthcare providers administering vaccine (vaccination providers) emergency use authorization (EUA) of the Moderna COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19): primary series for 12 years and older. Cambridge: Moderna, Inc.; 2022 [updated December 8, 2022]. https://eua.modernatx.com/covid19vaccine-eua/eua-fact-sheet-providers.pdf.
Pfizer/BioNTech. Fact sheet for healthcare providers administering vaccine (vaccination providers) emergency use authorization (EUA): Pfizer-BioNTech COVID-19 vaccine, bivalent (original and Omicron BA.4/BA.5) and booster dose for 12 years of age and older. New York: Pfizer/BioNTech; 2022 [updated December 8, 2022]. https://labeling.pfizer.com/ShowLabeling.aspx?id=14471&format=pdf.
Tahtinen S, Tong AJ, Himmels P, Oh J, Paler-Martinez A, Kim L, et al. IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nat Immunol. 2022;23(4):532–42.
Zhang L, More KR, Ojha A, Jackson CB, Quinlan BD, Li H, et al. Effect of mRNA-LNP components of two globally-marketed COVID-19 vaccines on efficacy and stability. NPJ Vaccines. 2023;8(1):156.
Cari L, Naghavi Alhosseini M, Mencacci A, Migliorati G, Nocentini G. Differences in the expression levels of SARS-CoV-2 spike protein in cells treated with mRNA-based COVID-19 vaccines: a study on vaccines from the real world. Vaccines (Basel). 2023;11(4):879.
Coleman BL, Sanderson R, Haag MDM, McGovern I. Effectiveness of the MF59-adjuvanted trivalent or quadrivalent seasonal influenza vaccine among adults 65 years of age or older, a systematic review and meta-analysis. Influenza Other Respir Viruses. 2021;15(6):813–23.
Lee JKH, Lam GKL, Shin T, Samson SI, Greenberg DP, Chit A. Efficacy and effectiveness of high-dose influenza vaccine in older adults by circulating strain and antigenic match: an updated systematic review and meta-analysis. Vaccine. 2021;39(Suppl 1):A24–35.
Wang X, Haeussler K, Spellman A, Phillips LE, Ramiller A, Bausch-Jurken MT, et al. Comparative effectiveness of mRNA-1273 and BNT162b2 COVID-19 vaccines in immunocompromised individuals: a systematic review and meta-analysis using the GRADE framework. Front Immunol. 2023;14:1204831.
Public Health Agency of Canada, National Advisory Committee on Immunization (NACI): Guidelines for the economic evaluation of vaccination programs in Canada, 1st edn. April 2023. https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/methods-process/incorporating-economic-evidence-federal-vaccine-recommendations/guidelines-evaluation-vaccination-programs-canada.html#Comparators.
Commonwealth of Australia, Department of Health: Guidelines for preparing a request for advice from the Australian Technical Advisory Group on Immunisation (ATAGI) to support Pharmaceutical Benefits Advisory Committee (PBAC) consideration of vaccines (Version 3 [Final]) February 2019. https://www.health.gov.au/sites/default/files/documents/2020/05/atagi-pre-submission-advice-for-industry-sponsors-wishing-to-make-a-pbac-submission-guidelines_0.pdf.
Commonwealth of Australia, Department of Health: Guidelines for preparing a submission to the Pharmaceutical Benefits Advisory Committee (Version 5.0) September 2016. https://pbac.pbs.gov.au/.
Chicoye A, Crepey P, Nguyen VH, Marquez-Pelaez S, Postma M, Pugliese A, et al. Contributions of cost-effectiveness analyses (CEA) to influenza vaccination policy for older adults in Europe. Vaccine. 2023;41(38):5518–24.
Postma M, Fisman D, Giglio N, Marquez-Pelaez S, Nguyen VH, Pugliese A, et al. Real-world evidence in cost-effectiveness analysis of enhanced influenza vaccines in adults ≥ 65 years of age: literature review and expert opinion. Vaccines (Basel). 2023;11(6):1089.
Schünemann H, Brożek J, Guyatt G, Oxman A (eds). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. The GRADE Working Group; 2013. https://gdt.gradepro.org/app/handbook/handbook.html.
Ahmed F, Temte JL, Campos-Outcalt D, Schünemann HJ. Methods for developing evidence-based recommendations by the Advisory Committee on Immunization Practices (ACIP) of the U.S. Centers for Disease Control and Prevention (CDC). Vaccine. 2011;29(49):9171–6.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
WHO COVID-19 Research Database: user guide and information: World Health Organization; 2023 [updated September 1, 2023]. https://www.who.int/publications/m/item/quick-search-guide-who-covid-19-database#News.
BC COVID Therapeutics Committee. Practice tool #2—definitions of CEV/immunosuppressed British Columbia, Canada: BC Centre for Disease Control; 2022 [updated July 25, 2022]. http://www.bccdc.ca/Health-Professionals-Site/Documents/COVID-treatment/PracticeTool2_CEVCriteria.pdf.
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
VanderWeele TJ. Optimal approximate conversions of odds ratios and hazard ratios to risk ratios. Biometrics. 2020;76(3):746–52.
Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–91.
Daly C, Anwer S, Welton NJ, Dias S, Ades A. Meta-analysis of event outcomes: guideline methodology document 3: NICE Guidelines Technical Support Unit; 2021. https://www.bristol.ac.uk/population-health-sciences/centres/cresyda/mpes/nice/guideline-methodology-documents-gmds/.
Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046–55.
Sterne JAC, Egger M. Regression methods to detect publication and other bias in meta-analysis. In: Rothstein HR, Sutton AJ, Borenstein M (eds); 2005.
Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for systematic reviews of interventions version 6.4 (updated August 2023): Cochrane; 2023. http://www.training.cochrane.org/handbook. Accessed 28 Feb 2024.
Schwarzer G. meta: an R package for meta-analysis. R News. 2007;7(3):40–5.
Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48.
Anderegg N, Althaus CL, Colin S, Hauser A, Laube A, Mausezahl M, et al. Assessing real-world vaccine effectiveness against severe forms of SARS-CoV-2 infection: an observational study from routine surveillance data in Switzerland. Swiss Med Wkly. 2022;152:w30163.
Breznik JA, Rahim A, Kajaks T, Hagerman M, Bilaver L, Colwill K, et al. Protection from omicron infection in residents of nursing and retirement homes in Ontario, Canada. J Am Med Dir Assoc. 2023;24(5):753–8.
Grewal R, Kitchen SA, Nguyen L, Buchan SA, Wilson SE, Costa AP, et al. Effectiveness of a fourth dose of covid-19 mRNA vaccine against the omicron variant among long term care residents in Ontario, Canada: test negative design study. BMJ. 2022;378:e071502.
Butt AA, Talisa VB, Yan P, Shaikh OS, Omer SB, Mayr FB. Vaccine effectiveness of 3 versus 2 doses of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccines in a high-risk national population. Clin Infect Dis. 2022;75(1):e579–84.
Hatfield KM, Baggs J, Wolford H, Fang M, Sattar AA, Montgomery KS, et al. Effectiveness of coronavirus disease 2019 (COVID-19) vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection among residents of US nursing homes before and during the delta variant predominance, December 2020–November 2021. Clin Infect Dis. 2022;75(Suppl 2):S147–54.
Kelly JD, Leonard S, Hoggatt KJ, Boscardin WJ, Lum EN, Moss-Vazquez TA, et al. Incidence of severe COVID-19 illness following vaccination and booster with BNT162b2, mRNA-1273, and Ad26.COV2.S vaccines. JAMA. 2022;328(14):1427–37.
Moline HL, Whitaker M, Deng L, Rhodes JC, Milucky J, Pham H, et al. Effectiveness of COVID-19 vaccines in preventing hospitalization among adults aged ≥ 65 years—COVID-NET, 13 states, February–April 2021. MMWR Morb Mortal Wkly Rep. 2021;70(32):1088–93.
Puranik A, Lenehan PJ, Silvert E, Niesen MJM, Corchado-Garcia J, O’Horo JC, et al. Comparative effectiveness of mRNA-1273 and BNT162b2 against symptomatic SARS-CoV-2 infection. Med. 2022;3(1):28–41 e8.
Robles-Fontan MM, Nieves EG, Cardona-Gerena I, Irizarry RA. Effectiveness estimates of three COVID-19 vaccines based on observational data from Puerto Rico. Lancet Reg Health Am. 2022;9: 100212.
Rosenberg ES, Dorabawila V, Easton D, Bauer UE, Kumar J, Hoen R, et al. Covid-19 vaccine effectiveness in New York state. N Engl J Med. 2022;386(2):116–27.
Thompson MG, Stenehjem E, Grannis S, Ball SW, Naleway AL, Ong TC, et al. Effectiveness of Covid-19 vaccines in ambulatory and inpatient care settings. N Engl J Med. 2021;385(15):1355–71.
Weng CH, Zhou S, Saal A, Hafferty M, McGuire DC, Chan PA. BNT162b2 and mRNA-1273 vaccine effectiveness against SARS-CoV-2 and variants in the urban underserved population. R I Med J. 2023;106(1):26–8.
Lin DY, Gu Y, Wheeler B, Young H, Holloway S, Sunny SK, et al. Effectiveness of Covid-19 vaccines over a 9-month period in North Carolina. N Engl J Med. 2022;386(10):933–41.
Hung Nguyen V, Boileau C, Bogdanov A, Sredl M, Bonafede M, Ducruet T, et al. Relative effectiveness of BNT162b2, mRNA-1273, and Ad26.COV2.S vaccines and homologous boosting in preventing COVID-19 in adults in the US. Open Forum Infect Dis. 2023;10(7):ofad288.
Braeye T, Catteau L, Brondeel R, van Loenhout JAF, Proesmans K, Cornelissen L, et al. Vaccine effectiveness against transmission of alpha, delta and omicron SARS-COV-2-infection, Belgian contact tracing, 2021–2022. Vaccine. 2023;41(20):3292–300.
Lytras T, Kontopidou F, Lambrou A, Tsiodras S. Comparative effectiveness and durability of COVID-19 vaccination against death and severe disease in an ongoing nationwide mass vaccination campaign. J Med Virol. 2022;94(10):5044–50.
Voko Z, Kiss Z, Surjan G, Surjan O, Barcza Z, Palyi B, et al. Nationwide effectiveness of five SARS-CoV-2 vaccines in Hungary-the HUN-VE study. Clin Microbiol Infect. 2022;28(3):398–404.
Voko Z, Kiss Z, Surjan G, Surjan O, Barcza Z, Wittmann I, et al. Effectiveness and waning of protection with different SARS-CoV-2 primary and booster vaccines during the delta pandemic wave in 2021 in Hungary (HUN-VE 3 Study). Front Immunol. 2022;13:919408.
Starrfelt J, Danielsen AS, Buanes EA, Juvet LK, Lyngstad TM, Ro GOI, et al. Age and product dependent vaccine effectiveness against SARS-CoV-2 infection and hospitalisation among adults in Norway: a national cohort study, July–November 2021. BMC Med. 2022;20(1):278.
van Ewijk CE, Kooijman MN, Fanoy E, Raven SF, Middeldorp M, Shah A, et al. COVID-19 vaccine effectiveness against SARS-CoV-2 infection during the Delta period, a nationwide study adjusting for chance of exposure, the Netherlands, July to December 2021. Euro Surveill. 2022;27(45):2200217.
Chico-Sanchez P, Gras-Valenti P, Algado-Selles N, Jimenez-Sepulveda N, Vanaclocha H, Peiro S, et al. The effectiveness of mRNA vaccines to prevent SARS-CoV-2 infection and hospitalisation for COVID-19 according to the time elapsed since their administration in health professionals in the Valencian Autonomous Community (Spain). Prev Med. 2022;163:107237.
Martinez-Baz I, Trobajo-Sanmartin C, Miqueleiz A, Guevara M, Fernandez-Huerta M, Burgui C, et al. Product-specific COVID-19 vaccine effectiveness against secondary infection in close contacts, Navarre, Spain, April to August 2021. Euro Surveill. 2021;26(39):2100894.
Kissling E, Hooiveld M, Martinez-Baz I, Mazagatos C, William N, Vilcu AM, et al. Effectiveness of complete primary vaccination against COVID-19 at primary care and community level during predominant Delta circulation in Europe: multicentre analysis, I-MOVE-COVID-19 and ECDC networks, July to August 2021. Euro Surveill. 2022;27(21):2101104.
Kim L, Garg S, O’Halloran A, Whitaker M, Pham H, Anderson EJ, et al. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-NET). Clin Infect Dis. 2021;72(9):e206–14.
Grohskopf LA, Blanton LH, Ferdinands JM, Chung JR, Broder KR, Talbot HK, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices—United States, 2022–23 influenza season. MMWR Recomm Rep. 2022;71(1):1–28.
Domnich A, de Waure C. Comparative effectiveness of adjuvanted versus high-dose seasonal influenza vaccines for older adults: a systematic review and meta-analysis. Int J Infect Dis. 2022;122:855–63.
Bhatt A. Real-world data in COVID-19 pandemic: an essential unmet health-care need. Perspect Clin Res. 2020;11(3):103–5.
Robert Koch Institute. STIKO: Aktualisierung der Influenza-Impfempfehlung für Personen im Alter von ≥60 Jahren. Epidemiologisches Bulletin. 2021(January).
Higgins JP. Commentary: heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol. 2008;37(5):1158–60.
Bello-Chavolla OY, Antonio-Villa NE, Valdes-Ferrer SI, Fermin-Martinez CA, Fernandez-Chirino L, Vargas-Vazquez A, et al. Effectiveness of a nationwide COVID-19 vaccination program in Mexico against symptomatic COVID-19, hospitalizations, and death: a retrospective analysis of national surveillance data. Int J Infect Dis. 2023;129:188–96.
Chemaitelly H, Ayoub HH, AlMukdad S, Coyle P, Tang P, Yassine HM, et al. Protection from previous natural infection compared with mRNA vaccination against SARS-CoV-2 infection and severe COVID-19 in Qatar: a retrospective cohort study. Lancet Microbe. 2022;3(12):e944–55.
Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25(20):3443–57.
Oh I-S. Beyond meta-analysis: secondary uses of meta-analytic data. Annu Rev Organ Psychol Organ Behav. 2020;7:125–53.
Grana C, Ghosn L, Evrenoglou T, Jarde A, Minozzi S, Bergman H, et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst Rev. 2022;12(12):CD015477.
Sadeghi S, Kalantari Y, Shokri S, Fallahpour M, Nafissi N, Goodarzi A, et al. Immunologic response, efficacy, and safety of vaccines against COVID-19 infection in healthy and immunosuppressed children and adolescents aged 2–21 years old: a systematic review and meta-analysis. J Clin Virol. 2022;153:105196.
Feikin DR, Higdon MM, Abu-Raddad LJ, Andrews N, Araos R, Goldberg Y, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399(10328):924–44.
Asghar N, Mumtaz H, Syed AA, Eqbal F, Maharjan R, Bamboria A, et al. Safety, efficacy, and immunogenicity of COVID-19 vaccines; a systematic review. Immunol Med. 2022;45(4):225–37.
Rahmani K, Shavaleh R, Forouhi M, Disfani HF, Kamandi M, Oskooi RK, et al. The effectiveness of COVID-19 vaccines in reducing the incidence, hospitalization, and mortality from COVID-19: a systematic review and meta-analysis. Front Public Health. 2022;10:873596.
Korang SK, von Rohden E, Veroniki AA, Ong G, Ngalamika O, Siddiqui F, et al. Vaccines to prevent COVID-19: a living systematic review with Trial Sequential Analysis and network meta-analysis of randomized clinical trials. PLoS ONE. 2022;17(1):e0260733.
Mohammed I, Nauman A, Paul P, Ganesan S, Chen KH, Jalil SMS, et al. The efficacy and effectiveness of the COVID-19 vaccines in reducing infection, severity, hospitalization, and mortality: a systematic review. Hum Vaccin Immunother. 2022;18(1):2027160.
Zheng C, Shao W, Chen X, Zhang B, Wang G, Zhang W. Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int J Infect Dis. 2022;114:252–60.
Au WY, Cheung PP. Effectiveness of heterologous and homologous covid-19 vaccine regimens: living systematic review with network meta-analysis. BMJ. 2022;377:e069989.
Lv J, Wu H, Xu J, Liu J. Immunogenicity and safety of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine: a systematic review. Infect Dis Poverty. 2022;11(1):53.
Iheanacho CO, Eze UIH, Adida EA. A systematic review of effectiveness of BNT162b2 mRNA and ChAdOx1 adenoviral vector COVID-19 vaccines in the general population. Bull Natl Res Cent. 2021;45(1):150.
Sharif N, Alzahrani KJ, Ahmed SN, Dey SK. Efficacy, immunogenicity and safety of COVID-19 vaccines: a systematic review and meta-analysis. Front Immunol. 2021;12:714170.
Rotshild V, Hirsh-Raccah B, Miskin I, Muszkat M, Matok I. Comparing the clinical efficacy of COVID-19 vaccines: a systematic review and network meta-analysis. Sci Rep. 2021;11(1):22777.
Chen M, Yuan Y, Zhou Y, Deng Z, Zhao J, Feng F, et al. Safety of SARS-CoV-2 vaccines: a systematic review and meta-analysis of randomized controlled trials. Infect Dis Poverty. 2021;10(1):94.
Kow CS, Hasan SS. Real-world effectiveness of BNT162b2 mRNA vaccine: a meta-analysis of large observational studies. Inflammopharmacology. 2021;29(4):1075–90.
Medical Writing Assistance.
Writing assistance was provided by Erin Bekes, PhD, and Sheri Arndt, PharmD, of ICON (Blue Bell, PA, USA) in accordance with Good Publication Practice (GPP3) guidelines, funded by Moderna, Inc., and under the direction of the authors.
Funding
This study was funded by Moderna, Inc. Authors employed by Moderna, Inc., were involved in the study design, analysis and interpretation of data, the writing of the manuscript, and the decision to submit the manuscript for publication. The sponsor funded the journal Rapid Service fee associated with the publication of this work.
Author information
Authors and Affiliations
Contributions
Ekkehard Beck, Katrin Haeussler, Xuan Wang, and Nathan Green designed and performed the systematic literature review and meta-analysis and critically evaluated the manuscript. Sushma Kavikondala, Maria Nassim, Nitendra Kumar Mishra, Mia Malmenäs, and Pawana Sharma performed the systematic literature review and meta-analysis and critically evaluated the manuscript. Ekkehard Beck, Mary T. Bausch-Jurken, and Nicolas Van de Velde conceptualized the article and provided oversight and critical evaluation of the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of Interest
Mary T. Bausch-Jurken, Nicolas Van de Velde, and Ekkehard Beck are employees of Moderna, Inc., and hold stock/stock options in the company. Katrin Haeussler, Xuan Wang, Maria Nassim, Nitendra Kumar Mishra, Mia Malmenäs, and Pawana Sharma are employees of ICON plc, a clinical research organization paid by Moderna, Inc., to conduct the study. Sushma Kavikondala is a former employee of ICON plc. Nathan Green is an independent consultant employed at University College of London, and was paid by Moderna, Inc., to conduct aspects of this study.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Prior publication: The work described herein has not been previously published in a peer-reviewed journal. An article preprint was posted on medRxiv on November 22, 2023, prior to peer review (https://doi.org/10.1101/2023.11.21.23298832).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kavikondala, S., Haeussler, K., Wang, X. et al. Comparative Effectiveness of mRNA-1273 and BNT162b2 COVID-19 Vaccines Among Older Adults: Systematic Literature Review and Meta-Analysis Using the GRADE Framework. Infect Dis Ther 13, 779–811 (2024). https://doi.org/10.1007/s40121-024-00936-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-024-00936-z