Abstract
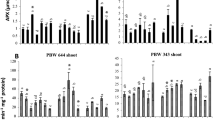
Drought-stressed plants accumulate cyclitols such as myo-inositol, pinitol, quercitol in the cytosol. These solutes (compatible solutes) protect plants from stress effects. Synthetic myo-inositol was used in the investigation of drought stress tolerance in pepper plants. Hydrogen peroxide (H2O2), membrane damage, ascorbate peroxidase (AP), catalase (CAT), proline and calcium increased in plants under drought conditions. Water status, calcium level, glutathione reductase activities increased in myo-inositol treated Capsicum annuum L. (pepper) under drought stress. Exogenous myo-inositol significantly decreased H2O2, membrane damage and proline levels and AP (except for 5 µM) and CAT activity, compared with untreated plants. Myo-inositol can play a role as effective as proline in signal transduction and in regulating concentrations of reactive oxygen species within tolerable ranges and in maintaining cell turgor by binding water molecules. Myo-inositol may become a useful instrument to eliminate the negative effects of drought environments.




Similar content being viewed by others
Abbreviations
- H2O2 :
-
Hydrogen peroxide
- SOD:
-
Superoxide dismutase
- GR:
-
Glutathione reductase
- AP:
-
Ascorbate peroxidase
- CAT:
-
Catalase
References
Aebi HE (1983) Catalase. In: Bergmeyer HU, Bergmeyer J, Grabl M (eds) Methods of enzymatic analysis. Verlag Chemie, Weinheim, pp 273–286
Araghi SG, Assad MT (1998) Evaluation of four screening techniques for drought resistance and their relationship to yield reduction ratio in wheat. Euphytica 103:293–299
Ashraf MPJC., Harris PJC (2004) Potential biochemical indicators of salinity tolerance in plants. Plant Sci 166:3–16
Azevedo RA, Alas RM, Smith RJ, Lea PJ (1998) Response of antioxidant enzymes to transfer from elevated carbon dioxide to air and ozone fumigation, in the leaves and roots of wild-type and a catalase-deficient mutant of barley. Physiol Plant 104:280–292
Barrs HD (1968) Determination of water deficits in plant tissue. In: Kozlowski TT (ed) Water deficits and plant growth, Academic press, Cambridge, pp 235–368
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Beyer WF, Fridowich I (1987) Assaying for superoxide dismutase activity: some large con- sequences of minor changes in conditions. Anal Biochem 161:559–566
Boguszewska D, Zagdańska B (2012) ROS as signaling molecules and enzymes of plant response to unfavorable environmental conditions. Oxidative stress—molecular mechanisms and biological effects. In: InTech. Rijeka, Croatia. https://doi.org/10.5772/33589
Bohnert HJ, Jensen RG (1996) Strategies for engineering water-stress tolerance in plants. Trends Biotechnol 14:89–97
Bonnet M, Camares O, Veisserie P (2000) Effects of zinc and influence of Acremonium lolii on growth parameters, chlorophyll a flurescence and antioxidant enzyme activities of ryegrass. J Exp Bot 5:945–953
Bonnet A, Jones W, Motherwell WDS (2006) Myo-inositol dehydrate: a redetermination. Acta Chrystalogr E 62:2902–2904
Carlberg I, Mannervik B (1985) Glutathion Reductase. Methods Enzymol 113:484–490
Cevik S, Yıldızlı A et al (2014) Some synthetic cyclitol derivatives alleviate the effect of water deficit in cultivated and wild-type chickpea species. J Plant Physiol 171:807–816
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought-from genes to the whole plant. Funct Plant Biol 30:239–264
Chimenti CA, Pearson J, Hall AJ (2002) Osmotic adjustment and yield maintenance under drought in sunflower. Field Crops Res 75:235–246
Davis BJ (1964) Disc electrophoresis-II method and application to human serum proteins. Ann NY Acad Sci 121:404–427
Demirevska K, Simova-Stoilova L, Vassileva V, Vaseva I, Grigorova B, Feller U (2008) Drought-induced leaf protein alterations in sensitive and tolerant wheat varieties. Gen Appl Plant Physiol 34:79–102
DeWal DB, Torabinejad J, Jones CA, Shope JC, Cangelosi AR, Thompson JE, Prestwich GD, Hama H (2001) Rapid accumulation of phosphatidylinositol 4,5-biphosphate and inositol 1,4,5-trisphosphate correlates with calcium mobilization in salt-stressed Arabidopsis. Plant Physiol 126:759–769
Dhanda SS, Sethi GS (1998) Inheritance of excised-leaf water loss and relative water content in bread wheat (Triticum aestivum). Euphytica 104:39–47
Engstrom EM, Ehrhardt DW et al (2002) Pharmacological analysis of nod factor-induced calcium spiking in Medicago truncatula evidence for the requirement of type IIA calcium pumps and phosphoinositide signaling. Plant Physiol 128:1390–1401
Fan HF, Ding L, Xu YL, Du CX (2017) Antioxidant system and photosynthetic characteristics responses to short-term PEG-induced drought stress. Russ J Plant Physiol 64(2):162–173
Filippou P, Antoniou C, Fotopoulos V (2011) Effect of drought and rewatering on the cellular status and antioxidant response of Medicago truncatula plants. Plant Signal Behav 6(2):270–277
Gadjev I, Ston JM, Gechev TS (2008) Programmed cell death in plants: new insights into redox regulation and the role of hydrogen peroxide. Int Rev Cell Mol Biol 270:87–144
Garcia PM, Hayashi AH, Silva EA, Figueiredo-Ribeiro RC, Carvalho MAM (2015) Structural and metabolic changes in rhizophores of the Cerrado species Chrysolaena abovata (Less.) Dematt. As influenced by drought and re-watering. Front Plant Sci 6:721
Gill SS, Tuteja (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Glaubitz U, Li X, Schaedel S, Erban A, Sulpice R, Kopka J, Hincha DK, Zuther E (2017) Integrated analysis of rice transcriptomic and metabolomics responses to elevated night temperatures identifies sensitivity and tolerance-related profiles. Plant Cell Environ 40:121–137
Hare PD, Cress WA (1997) Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul 21:79–102
Hossain MA, Hoque MA, Burrit DJ, Fujita M (2014) Proline Protects Plants Against Abiotic Oxidative Stress: Biochemical and Molecular Mechanisms. In: Ahmad P (ed) Oxidative damage to plants, Academic press, Cambridge, pp 477–522
Hsu SY, Hsu YT, Kao CH (2003) The effect of polyethylene glycol on proline accumulation in rice leaves. Biol Plant 46:73–78
Joshi R, Ramanarao MV, Baisakh N (2013) Arabidopsis plants constitutively overexpressing a myo-inositol 1-phosphate synthase gene (SaINO1) from the halophyte smooth cordgrass exhibits enhanced level of tolerance to salt stress. Plant Physiol Biochem 65:61–66
Kahlaoui B, Hachicha M, Misle E, Fidalgo F, Teixeira J (2018) Physiological and biochemical responses to the exogenous application of proline of tomato plants irrigated with saline water. J Saudi Soc Agric Sci 17(1):17–23
Kanchiswamy CN, Malnoy M, Occhipinti A, Maffei ME (2014) Calcium imaging perspectives in plants. Int J Mol Sci 15:3842–3859
Khunara N, Chauhan H, Kunara P (2012) Expression analysis of a heat-inducible, myo-inositol-1-phosphate synthase (MIPS) gene from wheat and the alternatively spliced variants of rice and Arabidopsis. Plant Cell Rep 31(1):237–251
Kishor PBK, Sangam S, Amruthal RN, Sri Laxmi P, Naidu KR, Rao KRSS., Rao S et al (2005) Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: its implications in plant growth and abiotic stress tolerance. Curr Sci 88(3):424–438
Knight H, Trewavas AJ, Knight MR (1997) Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J 12:1067–1078
Levitt J (1972) Responses of plants to environmental stresses. Academic press, New York, p 698
Lichtenthaler HK (1996) Vegetation stress: an introduction to the stress concept in plants. J Plant Physiol 148:4–14
Ma YH, Ma FW, Wang Y, Zhang J (2011) The responses of the enzymes related with ascorbate–glutathione cycle during drought stress in apple leaves. Acta Physiol Plant 33:173–180
Mani S, Van de Cotte B et al (2002) Altered levels of proline dehydrogenase cause hypersensitivity to proline and its analogs in Arabidopsis. Plant Physiol 128:73–83
Marchant A, Tausz M et al (2006) Cyclitols and carbohydrates in leaves and roots of 13 eucalyptus species suggest contrating physiological responses to water deficit. Plant Cell Environ 29:2017–2029
Mechri B, Tekaya M, Cheheb H, Hammami M (2015) Determination of mannitol sorbitol and myo-inositol in olive tree roots and rhizospheric soil by gas chromatography and effect of severe drought conditions on their profiles. J Chromatogr Sci 53(10):1631–1638
Mittler R, Zilinskas BA (1993) Detection of ascorbate peroxidase activity in native gels by inhibition of the ascorbate-dependent reduction of nitroblue tetrazolium. Anal Biochem 212:540–546
Moghaieb REA, Tanaka N et al (2006) Characterization of salt tolerance in ectoine-transformed tobacco plants (Nicotiana tabaccum): photosynthesis, osmotic adjustment, and nitrogen partitioning. Plant Cell Environ 29:173–182
Munnik T, Vermeer JE (2010) Osmotic stress-induced phosphoinositide and inositol phosphate signalling in plants. Plant Cell Environ 33:655–669
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Öztürk L, Demir Y (2002) In vivo and in vitro protective role of proline. Plant Growth Regul 38:259–264
Per TS, Khan NA, Reddy PS, Masood A, Hasanuzzaman M, Khan MIR, Anjum NA (2017) Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: phytohormones, mineral nutrients and transgenics. Plant Physiol Biochem 115:126–140
Perera IY, Hung CY, Moore C et al (2008) Transgenic Arabidopsis plants expressing the type 1 inositol 5-phosphatase and altered abscisic acid signalling. Plant Cell 20(10):2876–2893
Posternak T (1965) The cyclitols. In: Leeder E (ed) Chemistry, biochemistry, biology. Hermann, Paris, pp 284–286
Reddy AR, Chaitanya KV, Vivekananda M (2004) Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol 161: 1189–1202
Sambe MAH, He X, Tu Q, Guo Z (2015) A cold-induced myo-inositol transporter-like gene confers tolerance to multiple abiotic stresses in transgenic tobacco plants. Physiol Plant 153:355–364
Sanchez-Rodriguez E, Rubio-Wilhemi MM, Cervilla UM et al (2010) Genotypic differences in some physiological parameters symptomatic for oxidative stress under moderate drought in tomato plants. Plant Sci 178:30–40
Sanders D, Brownlee C, Harper JF (1999) Communicating with calcium. Plant Cell 11:691–706
Sanders D, Pelloux J, Brownlee C, Harper JF (2002) Calcium at the crossroads of signalling. Plant Cell 14:401–417
Scandalios JG (1993) Oxygen stress and superoxide dismutases. Plant Physiol 101:7
Serraj R, Sinclair TR (2002) Osmolyte accumulation: can it really help increase crop yield under drought conditions? Plant Cell Environ 25:333–341
Sharma P, Dubey RS (2005) Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regul 46:209–221
Srinivas V, Balasubramanian D (1995) Proline is a protein-compatible hydrotrope. Langmuir 11:2830–2833
Stevenson-Paulik J et al (2005) Generation of phytate-free seeds in Arabidopsis through disruption of inositol polyphosphate kinases. Proc Natl Acad Sci 102: 12612–12617
Steward CR, Hanson AD (1980) Proline accumulation as a metabolic response to water stress. In: Turner NC, Kramer PJ (eds). Wiley, New York, pp 173–189
Szabados L, Kovacs H, Zilberstein A, Bouchereau A (2011) Plants in extreme environments: importance of protective compounds in stress tolerance. Adv Bot Res 57:105–150
Tan BH, Halloran GM (1982) Variation and correlations of proline accumulation in spring wheat cultivars. Crop Sci 22:459–463
Tang EHC, Leung FP et al (2007) Calcium and reactive oxygen species increase in endothelial cells in response to releasers of endothelium-derived contracting factor. Br J Pharmacol 151:15–23
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley powdery mildew interaction. Plant J 11(6):1187–1194
Tucker EB, Boss WF (1996) Mastoparan-induced intracellular Ca2+ fluxes may regulate cell-to-cell communication in plants. Plant Physiol 111:459–467
Valluru R, Van den Ende W (2011) Myo-inositol and beyond—emerging networks under stress. Plant Sci 181:387–400
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66
Vieira EA, Silva MG, Moro CF, Laura VA (2017) Physiological and biochemical changes attenuate the effects of drought on the Cerrado species Vatairea macrocarpa (Benth.) Ducke. Plant Physiol Biochem 115:472–483
Vitoria A, Lea P, Azevedo PA (2001) Antioxidant enzymes responses to cadmium in radish tissues. Phytochemistry 57(5):701–710
Wanek W, Richter A (1997) Biosynthesis and accumulation of D-ononitol in Vigna umbellata in response to drought stress. Physiol Plant 101:416–424
Woodbury W, Spencer AK, Stahmann MA (1971) An improved procedure using ferricyanide for detecting catalase isozymes. Anal Biochem 44:301–305
Wu QS, Xia RX (2006) Arbuscular mycorrhizal fungi influence growth, osmotic adjustment and photosynthesis of citrus under well-watered and water stress conditions. J Plant Physiol 163:417–425
Wu Y, Kuzma J, Marechal E, Graeff R, Lee HC, Foster R, Chua NH (1997) Abscisic acid signaling through cyclic ADP-ribose in plants. Science 278:2126–2130
Zarcinas BA, Cartwright B, Spouncer LR (1987) Nitric acid digestion and multi-element analysis of plant material by inductively coupled plasma spectrometry. Commun Soil Sci Plant Anal 18:131–146
Zhai S, Jinxi H, Lei X, Yanyan A, Shaozhen H, Quingchang L (2016) A myo-inositol-1-phosphate synthase gene, IbMIPS1, enhances salt and drought tolerance and stem nematode resistance in transgenic sweet potato. Plant Biotechnol J 14:592–602
Acknowledgements
The authors express their gratitude to Assoc.Prof.Dr. Riza BINZET for his assistance in Microscopic investigation of hydrogen peroxide analysis and to Assoc.Prof.Dr. Serdar GULTEKIN from Atatürk University for providing myo-inositol. This academic work was linguistically supported by the Mersin Technology Transfer Office Academic Writing Center of Mersin University. The present study was supported by grant from Mersin University Scientific Research Foundation (Grant no. BAP-FBE BB (AY) 2012-8).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Bartosz.
Rights and permissions
About this article
Cite this article
Yildizli, A., Çevik, S. & Ünyayar, S. Effects of exogenous myo-inositol on leaf water status and oxidative stress of Capsicum annuum under drought stress. Acta Physiol Plant 40, 122 (2018). https://doi.org/10.1007/s11738-018-2690-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-018-2690-z