Abstract
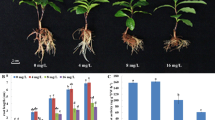
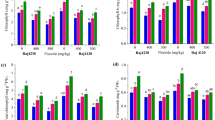
Tea (Camellia sinensis (L.) O. Kuntze) hyper-accumulates fluoride (F), mainly in the leaves. To understand how tea copes with the stress caused by F, we tracked photosynthesis, antioxidant defense, and cell ultrastructure under different F concentrations (0–50 mg L−1). High F (≥5 mg L−1) caused decreases in photosynthetic and chlorophyll fluorescence parameters. Activated oxygen metabolism was altered by F, as manifested in increasing lipid peroxidation, electrolyte leakage (EL), and accumulation of H2O2. The activities of ascorbate peroxidase (APX, EC 1.11.1.1) and catalase (CAT, EC 1.11.1.6) increased at 0–5 mg L−1 F, but sharply decreased less than 10–50 mg L−1 F. The activity of manganese superoxide dismutase (Mn-SOD, EC 1.15.1.1) decreased with increasing F concentration. Expression of genes encoding antioxidant enzymes were in accordance with their measured activities. The results suggest that the antioxidant enzymes in the tea plant can eliminate reactive oxygen species (ROS) at <5 mg L−1 F, but not at 20–50 mg L−1 F. High F increased the number of epidermal hairs on tea leaves and decreased the stomatal aperture, reducing water loss. The leaf cellular structure appeared normal under 1–50 mg L−1 F, although starch grains in chloroplast increased with increasing F. Proline and betaine play important roles in osmotic regulation in tea plant tolerating F stress. ROS scavenging and greater number of epidermal hairs are likely parts of the tea plant F-tolerance mechanism.











Similar content being viewed by others
References
Amin T, Yahya A, Maziah M, Ahmad S, Zakaria W (2012) Leaf water status, proline content, lipid peroxidation and accumulation of hydrogen peroxide in salinized Chinese kale (Brassica alboglabra). J Food Agric Environ 10:371–374
Arora A, Sairam R, Srivastava G (2002) Oxidative stress and antioxidative system in plants. Current Sci India 82:1227–1238
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141:391–396
Ashraf M, Foolad M (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Bhatia NP, Walsh KB, Baker AJ (2005) Detection and quantification of ligands involved in nickel detoxification in a herbaceous Ni hyperaccumulator Stackhousia tryonii Bailey. J Exp Bot 56:1343–1349
Bosabalidis AM, Kofidis G (2002) Comparative effects of drought stress on leaf anatomy of two olive cultivars. Plant Sci 163:375–379
Cai H, Peng C, Chen J, Hou R, Gao H, Wan X (2014) X-ray photoelectron spectroscopy surface analysis of fluoride stress in tea (Camellia sinensis (L.) O. Kuntze) leaves. J Fluorine Chem 158:11–15
Cakmak I, Horst WJ (1991) Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol Plantarum 83:463–468
Chakrabarti S, Patra PK (2013) Effect of fluoride on superoxide dismutase activity in four common crop plants. Fluoride 46:59–61
Dan TV, KrishnaRaj S, Saxena PK (2000) Metal tolerance of scented geranium (Pelargonium sp. ‘Frensham’): effects of cadmium and nickel on chlorophyll fluorescence kinetics. Int J Phytoremediat 2:91–104
Dat JF, Inzé D, Van Breusegem F (2001) Catalase-deficient tobacco plants: tools for in planta studies on the role of hydrogen peroxide. Redox Rep 6:37–42
Dey U, Mondal NK, Das K, Dattaa JK (2012) Dual effects of fluoride and calcium on the uptake of fluoride, growth physiology, pigmentation, and biochemistry of bengal gram seedlings (Cicer arietinum L.). Fluoride 45:389–393
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Elloumi N, Abdallah FB, Mezghani I, Rhouma A, Boukhris M, Tunisia S (2005) Effect of fluoride on almond seedlings in culture solution. Fluoride 38:193
Faisal M, Anis M (2009) Changes in photosynthetic activity, pigment composition, electrolyte leakage, lipid peroxidation, and antioxidant enzymes during ex vitro establishment of micropropagated Rauvolfia tetraphylla plantlets. Plant Cell Tiss Org 99:125–132
Fornasiero RB (2001) Phytotoxic effects of fluorides. Plant Sci 161:979–985
Fornasiero RB (2003) Fluorides effects on Hypericum perforatum plants: first field observations. Plant Sci 165:507–513
Gajić G, Mitrović M, Pavlović P, Stevanović B, Djurdjević L, Kostić O (2009) An assessment of the tolerance of Ligustrum ovalifolium Hassk. to traffic-generated Pb using physiological and biochemical markers. Ecotox Environ Safe 72:1090–1101
Gao H, Zhao Q, Zhang X, Wan X, Mao J (2014) Localization of fluoride and aluminum in subcellular fractions of tea leaves and roots. J Agr Food Chem 62:2313–2319
Gautam R, Bhardwaj N (2010) Bioaccumulation of fluoride in different plant parts of Hordeum vulgare (barley) var. rd-2683 from irrigation water. Fluoride 43:57–60
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Bioch 48:909–930
Guo Y, Zhou H, Zhang L (2006) Photosynthetic characteristics and protective mechanisms against photooxidation during high temperature stress in two citrus species. Sci Hortic 108:260–267
Gupta N, Meena S, Gupta S, Khandelwal S (2002) Gas exchange, membrane permeability, and ion uptake in two species of Indian jujube differing in salt tolerance. Photosynthetica 40:535–539
Gupta S, Banerjee S, Mondal S (2009) Phytotoxicity of fluoride in the germination of paddy (Oryza sativa) and its effect on the physiology and biochemistry of germinated seedlings. Fluoride 42:142
Islam E, Liu D, Li T, Yang X, Jin X, Mahmood Q, Tian S, Li J (2008) Effect of Pb toxicity on leaf growth, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi. J Hazard Mater 154:914–926
Jha S, Nayak A, Sharma Y (2009) Fluoride toxicity effects in onion (Allium cepa L.) grown in contaminated soils. Chemosphere 76:353–356
Karbassi P, Garrard L, West S (1971) Reversal of low temperature effects on a tropical plant by gibberellic acid. Crop Sci 11:755–757
Konishi S (1992) Promotive effects of aluminium on tea plant growth. Jpn Agrl Res Q 26:26–33
Kumar KA, Rao AVB (2008) Physiological responses to fluoride in two cultivars of mulberry. World J Agric Sci 4:463–466
Küpper H, Zhao F, McGrath SP (1999) Cellular compartmentation of zinc in leaves of the hyperaccumulator Thlaspi caerulescens. Plant Physiol 119:305–312
Lee S-H, Ahsan N, Lee K-W, Kim D-H, Lee D-G, Kwak S-S, Kwon S-Y, Kim T-H, Lee B-H (2007) Simultaneous overexpression of both CuZn superoxide dismutase and ascorbate peroxidase in transgenic tall fescue plants confers increased tolerance to a wide range of abiotic stresses. J Plant Physiol 164:1626–1638
Li Q, Yu L, Deng Y, Li W, Li M, Cao J (2007) Leaf epidermal characters of Lonicera japonica and Lonicera confuse and their ecology adaptation. J Forestry Res 18:103–108
Li C, Xu H, Xu J, Chun X, Ni D (2011a) Effects of aluminium on ultrastructure and antioxidant activity in leaves of tea plant. Acta Physiol Plant 33:973–978
Li C, Zheng Y, Zhou J, Xu J, Ni D (2011b) Changes of leaf antioxidant system, photosynthesis and ultrastructure in tea plant under the stress of fluorine. Biol Plantarum 55:563–566
Lin CC, Kao CH (2000) Effect of NaCl stress on H2O2 metabolism in rice leaves. Plant Growth Regul 30:151–155
Liu D, Kottke I (2004) Subcellular localization of copper in the root cells of Allium sativum by electron energy loss spectroscopy (EELS). Bioresour Technol 94:153–158
Liu Y, Zhang G, Qi M, Li T (2015) Effects of calcium on photosynthesis, antioxidant system, and chloroplast ultrastructure in tomato leaves under low night temperature stress. J Plant Growth Regul 1–11
Mackowiak C, Grossl P, Bugbee B (2003) Biogeochemistry of fluoride in a plant–solution system. J Environ Qual 32:2230–2237
McNeil SD, Rhodes D, Russell BL, Nuccio ML, Shachar-Hill Y, Hanson AD (2000) Metabolic modeling identifies key constraints on an engineered glycine betaine synthesis pathway in tobacco. Plant Physiol 124:153–162
Mishra S, Srivastava S, Tripathi R, Kumar R, Seth C, Gupta D (2006) Lead detoxification by coontail (Ceratophyllum demersum L.) involves induction of phytochelatins and antioxidant system in response to its accumulation. Chemosphere 65:1027–1039
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Pereira GJG, Molina SMG, Lea PJ, Azevedo RA (2002) Activity of antioxidant enzymes in response to cadmium in Crotalaria juncea. Plant Soil 239:123–132
Ruan J, Wong MH (2001) Accumulation of fluoride and aluminium related to different varieties of tea plant. Environ Geochem and Hlth 23:53–63
Ruan J, Ma L, Shi Y, Han W (2004) The impact of pH and calcium on the uptake of fluoride by tea plants (Camellia sinensis L.). Ann Bot 93:97–105
Saini P, Khan S, Baunthiyal M, Sharma V (2013) Effects of fluoride on germination, early growth and antioxidant enzyme activities of legume plant species Prosopis juliflora. J Environ Biol 34:205–209
Shu W, Zhang Z, Lan C, Wong M (2003) Fluoride and aluminium concentrations of tea plants and tea products from Sichuan Province, PR China. Chemosphere 52:1475–1482
Singh M, Verma KK (2013) Influence of fluoride-contaminated irrigation water on physiological responses of poplar seedlings (Populus deltoides L. clone-S 7 C 15). Fluoride 46:83–89
Smirnoff N, Cumbes QJ (1989) Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 28:1057–1060
Tingey DT, Hogsett WE (1985) Water stress reduces ozone injury via a stomatal mechanism. Plant Physiol 77:944–947
Weinstein LH, Davison A (2004) Fluorides in the environment: effects on plants and animals. CABI
Xie Z, Chen Z, Sun W, Guo X, Yin B, Wang J (2007) Distribution of aluminum and fluoride in tea plant and soil of tea garden in Central and Southwest China. Chinese Geogr Sci 17:376–382
Yamagishi M (1998) Effects of culture temperature on the enlargement, sugar uptake, starch accumulation, and respiration of in vitro bulblets of Lilium japonicum Thunb. Sci Hortic 73:239–247
Yang S, Miller G (1963) Biochemical studies on the effect of fluoride on higher plants. 1. Metabolism of carbohydrates, organic acids and amino acids. Biochem J 88:505
Yang D, Liu Y, Sun M, Zhao L, Wang Y, Chen X, Wei C, Gao L, Xia T (2012) Differential gene expression in tea (Camellia sinensis L.) calli with different morphologies and catechin contents. J Plant Physiol 169:163–175
Zhang X, Hu C, Yao J (2010) Tetraploidization of diploid Dioscorea results in activation of the antioxidant defense system and increased heat tolerance. J Plant Physiol 167:88–94
Zhang J, Jiang X, Li T, Cao X (2014a) Photosynthesis and ultrastructure of photosynthetic apparatus in tomato leaves under elevated temperature. Photosynthetica 52:430–436
Zhang Z, Ma M, Liu X, Ma R, Liu J (2014b) Structural and ultra-structural disorders in Ziziphus jujuba Miller fruits under fluorine stress. Fluoride 47:208–226
Acknowledgments
The present work was financially supported by the Earmarked Fund for Modern Agro-industry Technology Research System in Tea Industry (CARS-23, the Ministry of Agriculture of P. R. China), Anhui Major Demonstration Project for Leading Talent Team on Tea Chemistry and Health, Natural Science Foundation of Anhui Province (1408085MKL38), Anhui Scientific and Technological Project (1406C085017), and Changjiang Scholars and Innovative Research Team in University (IRT1101).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Krolicka.
Rights and permissions
About this article
Cite this article
Cai, H., Dong, Y., Li, Y. et al. Physiological and cellular responses to fluoride stress in tea (Camellia sinensis) leaves. Acta Physiol Plant 38, 144 (2016). https://doi.org/10.1007/s11738-016-2156-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-016-2156-0