Abstract
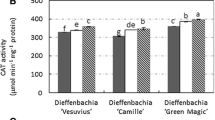
The effects of photosynthetic photon flux density (PPFD) on antioxidant metabolism and photosynthetic properties in leaves during ex vitro establishment of micropropagated Rauvolfia tetraphylla plantlets were investigated. In vitro-propagated plantlets were acclimatized at either 50 (Low-light = LL) or 300 (High-light = HL) μmol m−2s−1 photosynthetic PPFD for 4 weeks under controlled conditions. Increases in chlorophyll (Chl) a, b and carotenoid levels were observed in plantlets acclimatized at both light intensities. At transplantation, micropropagated plantlets were not photosynthetically active, but the net photosynthetic rate increased in newly formed leaves over time during acclimatization. The observed differences in pigment contents and photosynthetic rates suggested adaptation of plantlets from heterotrophic to autotrophic mode of nutrition during acclimatization. Changes in activities of antioxidant enzymes were also observed during acclimatization. Superoxide dismutase activity increased in plantlets acclimatized at HL intensities. Likewise, changes in activity of catalase and ascorbate peroxidase were also detected. These observed changes reflected the ability of plants in developing an antioxidant enzymatic defense system aiding in survival against oxidative stress and in reducing release of free radicals.





Similar content being viewed by others
References
Aebi H (1984) Catalase in vitro methods. Enzymology 105:121–126
Ali MB, Hahn EJ, Paek KY (2005) Effects of light intensities on antioxidant enzymes and malondialdehyde content during short-term acclimatization on micropropagated Phalaenopsis plantlet. Environ Exp Bot 54:109–120
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Asada K (2000) The water–water cycle as alternative photon and electron sinks. Philos Trans R Soc Lond B Biol Sci 355:1419–1431
Bailly C, Benamar A, Corbineau F, Dome D (1996) Changes in malondialdehyde content and in superoxide dismutase, catalase and glutathione reductase activities in sunflower seed as related to deterioration during accelerated aging. Physiol Plant 97:104–110
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein–dye-binding. Ann Biochem 72:248–254
Cakmak I, Horst WJ (1991) Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase and peroxidase activites in root tips of soybean (Glycine max). Physiol Plant 83:463–468
Carvalho LC, Amancio S (2002) Antioxidant defense system in plantlets transferred from in vitro to ex vitro: effects of increasing light intensity and CO2 concentration. Plant Sci 162:33–40
Chen GX, Asada K (1989) Ascorbate peroxidase in tea leaves: occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol 30:987–998
Desjardins Y (1995) Photosynthesis in vitro on the factors regulating CO2 assimilation in micropropagation systems. Acta Hortic 393:45–61
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Donnelly DJ, Vidaver WE (1984) Pigment content and gas exchange of red raspberry transferred in vitro and ex vitro. J Am Soc Hortic Sci 109:177–181
Faisal M, Ahmad N, Anis M (2005) Shoot multiplication in Rauvolfia tetraphylla L. using thidiazuron. Plant Cell Tissue Organ Cult 80:187–190
Foyer CH, Halliwell B (1976) The presence of glutathione reductase in chloroplast: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Foyer CH, Lelandais M, Kunert KJ (1994) Photooxidative stress in plants. Physiol Plant 92:696–717
Foyer CH, Lopez-Delgado H, Dat JF, Scott IM (1997) Hydrogen peroxide and glutathione-associated mechanisms of acclamatory stress tolerance and signaling. Physiol Plant 100:241–254
Huylenbroeck JMV, Debergh PC (1996) Impact of sugar concentration in vitro on photosynthesis and carbon metabolism during ex vitro acclimatization of Spathiphyllum plantlets. Physiol Plant 96:298–304
Huylenbroeck JMV, Piqueras A, Debergh PC (1998) Photosynthesis and carbon metabolism ion leaves formed prior and during acclimatization of micropropagated plants. Plant Sci 34:21–30
Huylenbroeck JMV, Piqueras A, Deberghm PC (2000) The evolution of photosynthetic capacity and the antioxidant enzymatic system during acclimatization of micropropagated Calathea plants. Plant Sci 155:59–66
Inove S, Kawanishi S (1987) Hydroxyl radical production and human DNA damage induced by ferric nitrotriacetate and hydrogen peroxide. Can Res 47:6522–6527
Ishikawa T, Yoshimura K, Sakai K, Tamoi M, Takeda T, Shigeoka S (1998) Molecular characterization and physiological role of a glyoxisomes-bound ascorbate peroxidase from spinach. Plant Cell Physiol 39:23–34
Jeon WW, Ali MB, Hahn EJ, Paek KY (2005) Effects of photon flux density on the morphology, photosynthesis and growth of a CAM orchid, Doritaenopsis during post-micropropagation acclimatization. Plant Growth Regul 45:139–147
Kenneth E, Pallet KE, Young J (2000) Carotenoids. In: Ruth GA, Hess JL (eds) Antioxidants in higher plants. CRC Press, USA, pp 60–81
Kyle ME, Farber JL (1991) Biochemical mechanism of toxic cell injury. In: Haschek MW, Rousseavx CG (eds) Toxicological pathology. Academic Press, San Diego, pp 81–85
Lutts S, Kinet JM, Bouharmont J (1995) Changes in plant response to NaCl during development of rice (Oryza sativa L.) varieties differing in salinity resistance. J Exp Bot 46:1843–1852
Mackinney G (1941) Absorption of light by chlorophyll solution. J Biol Chem 140:315–322
Maclachan S, Zalick S (1963) Plastid structure, chlorophyll concentration and free amino acid composition of chlorophyll mutant barley. Can J Bot 105:3–1062
Mittler R, Zilinskas BA (1994) Regulation of pea cytosolic ascorbate peroxidase and other antioxidant enzymes during the progression of drought stress and following recovery from drought. Plant J 5:397–405
Miyake C, Asada K (1996) Inactivation mechanism of ascorbate peroxidase at low concentrations of ascorbate; hydrogen peroxide decomposes compound I of ascorbate peroxidase. Plant Cell Physiol 37:423–430
Mundi JR, Sinha BK, Gianni L, Myers CE (1984) Hydroxyl radical production and DNA damage induced by anthracycline- iron complex. FEBS Lett 172:226–230
Munne-Bosch S, Alegre L (2002) Interplay between ascorbic acid and lipophilic antioxidant defences in chloroplasts of water-stressed Arabidopsis plants. FEBS Lett 524:145–148
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Ann Rev Plant Physiol 49:249–279
Noctor G, Gomez L, Vanacker H, Foyer CH (2002) Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signaling. J Exp Bot 53:1283–1304
Pospisilova J, Catsky J, Sestak Z (1997) Photosynthesis in plants cultivated in vitro. In: Pessaraki M (ed) Handbook of Photosynthesis. Marcel Dekker, New York, pp 525–540
Rao MV (1992) Cellular detoxifying mechanisms determine age dependent injury in tropical plants exposed to SO2. J Plant Physiol 140:733–740
Scandalios JG (1990) Response of plant antioxidant defense genes to environmental stress. Adv Genet 28:1–41
Smirnoff N, Wheeler GL (2000) Ascorbic acid in plants: biosynthesis and function. Crit Rev Plant Sci 19:267–290
Von Willert DJ, Matyssek R, Herppich W (1995) Experimentelle Pflanzenokologie. Gundlagem and Anwendungen, New York
Young AJ (1991) The protective role of carotenoids in higher plants. Physiol Plant 83:702–708
Zhang J, Kirkham MB (1996) Drought stress induced changes in activities of superoxide dismutase, catalase and peroxidase in wheat species. Plant Cell Physiol 35:785–791
Acknowledgments
The authors gratefully acknowledge the Department of Science and Technology, Government of India, New Delhi for the award of a SERC Fast Track Scheme (Project No. SR/FT/L-067/2006) to MF and for providing research support under DST-FIST (2005) programme. The authors are also thankful to Professor M. Iqbal, Jamia Hamdard, New Delhi and Dr. Conor O’Reilly, UCD School of Biology and Environmental Science, University College, Dublin for their critical reading and comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Faisal, M., Anis, M. Changes in photosynthetic activity, pigment composition, electrolyte leakage, lipid peroxidation, and antioxidant enzymes during ex vitro establishment of micropropagated Rauvolfia tetraphylla plantlets. Plant Cell Tiss Organ Cult 99, 125–132 (2009). https://doi.org/10.1007/s11240-009-9584-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-009-9584-0