Abstract
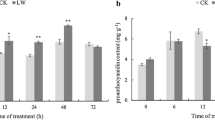
Tartary buckwheat (Fagopyrum tataricum) is rich in flavonoids. Anthocyanins, a special class of flavonoids, offer significant nutrients and provide the red pigment found in sprouts. The anthocyanins biosynthesis has been shown influenced by environmental stress. In this study, we investigate the effects of cold stress on anthocyanins biosynthesis of tartary buckwheat sprouts. On cellular level, cross-sectional observations were performed and result indicated that anthocyanins accumulated primarily in the epidermal and cortex cells of hypocotyls. Biochemical analysis, including anthocyanin quantification, thin-layer chromatography and radical scavenging assay, showed that cold stress significantly increased the synthesis of anthocyanins and antioxidant activity of tartary buckwheat sprouts. At the molecular level, semi-RT-PCR was used to investigate 14 anthocyanin-related genes in tartary buckwheat sprouts treated with cold stress. All the selected genes were upregulated in cold-stressed sprouts, except for FtGST, FtAHA, and FtMYB. More importantly, the expression level of three late biosynthesis genes, FtF3′H, FtDFR, and FtANS, was highest in hypocotyl tissue. Our results suggest that anthocyanins play a considerable role in the cold stress tolerance of tartary buckwheat sprouts.




Similar content being viewed by others
References
Alscher RG, Donahue JL, Cramer CL (1997) Reactive oxygen species and antioxidants: relationships in green cells. Physiol Plant 100:224–233
Ban Y, Honda C, Hatsuyama Y, Igarashi M, Bessho H, Moriguchi T (2007) Isolation and functional analysis of a MYB transcription factor gene that is a key regulator for the development of red coloration in apple skin. Plant Cell Physiol 48:958–970
Bravo L, Mateos R (2008) Analysis of flavonoids in functional foods and nutraceuticals. In: Hurst WJ (ed) Methods of analysis for functional foods and nutraceuticals. CRC Press, New York, pp 1–64
Chalker-Scott L (1999) Environmental significance of anthocyanins in plant stress responses. Photochem Photobiol 70:1–9
Chu H, Jeong JC, Kim WJ, Chung DM, Jeon HK, Ahn YO, Kim SH, Lee HS, Kwak SS, Kim CY (2012) Expression of the sweetpotato R2R3-type IbMYB1a gene induces anthocyanin accumulation in Arabidopsis. Physiol Plant 148:189–199
Cominelli E, Gusmaroli G, Allegra D, Galbiati M, Wade HK, Jenkins GI, Tonelli C (2008) Expression analysis of anthocyanin regulatory genes in response to different light qualities in Arabidopsis thaliana. J Plant Physiol 165:886–894
Crifò T, Petrone G, Lo Cicero L, Lo Piero AR (2011) Short cold storage enhances the anthocyanin contents and level of transcripts related to their biosynthesis in blood oranges. J Agric Food Chem 60:476–481
Deroles S (2009) Anthocyanin biosynthesis in plant cell cultures: a potentialsource of natural colourants. In: Kevin G et al (eds) Anthocyanins: biosynthesis, functions and applications. Springer, New York, pp 107–117
Gonzalez A, Zhao M, Leavitt JM, Lloyd AM (2008) Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J 53:814–827
Gould KS, Markham KR, Smith RH, Goris JJ (2000) Functional role of anthocyanins in the leaves of Quintinia serrata A. Cunn. J Exp Bot 51:1107–1115
Grotewold E (2006) The genetics and biochemistry of floral pigments. Annu Rev Plant Biol 57:761–780
Hichri I, Barrieu F, Bogs J, Kappel C, Delrot S, Lauvergeat V (2011) Recent advances in the transcriptional regulation of the flavonoid biosynthetic pathway. J Exp Bot 62:2465–2483
Kim DO, Lee KW, Lee HJ, Lee CY (2002) Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J Agric Food Chem 50:3713–3717
Kim SL, Kim SK, Park CH (2004) Introduction and nutritional evaluation of buckwheat sprouts as a new vegetable. Food Res Int 37:319–327
Kim SJ, Kawaharada C, Suzuki T, Saito K, Hashimoto N, Takigawa S, Noda T, Matsuura-Endo C, Yamauchi H (2006) Effect of natural light periods on rutin, free amino acid and vitamin C contents in the sprouts of common (Fagopyrum esculentum Moench) and tartary (F. tataricum Gaertn.) buckwheats. Food Sci Technol Res 12:199–205
Kim SJ, Zaidul ISM, Maeda T, Suzuki T, Hashimoto N, Takigawa S, Noda T, Matsuura-Endo C, Yamauchi H (2007) A time-course study of flavonoids in the sprouts of tartary (Fagopyrum tataricum Gaertn.) buckwheats. Sci Hortic 115:13–18
Kim SJ, Zaidul I, Suzuki T, Mukasa Y, Hashimoto N, Takigawa S, Noda T, Matsuura-Endo C, Yamauchi H (2008) Comparison of phenolic compositions between common and tartary buckwheat (Fagopyrum) sprouts. Food Chem 110:814–820
Kim HJ, Park KJ, Lim JH (2011) Metabolomic analysis of phenolic compounds in buckwheat (Fagopyrum esculentum M.) sprouts treated with methyl jasmonate. J Agric Food Chem 59:5707–5713
Lee B, Lee H, Xiong L, Zhu JK (2002) A mitochondrial complex I defect impairs cold-regulated nuclear gene expression. Plant Cell 14:1235–1251
Leng P, Itamura H, Yamamura H, Deng X (2000) Anthocyanin accumulation in apple and peach shoots during cold acclimation. Sci Hortic 83:43–50
Li C, Bai Y, Li S, Chen H, Han X, Zhao H, Shao J, Park S, Wu Q (2012a) Cloning, characterization, and activity analysis of a flavonol synthase gene FtFLS1 and its association with flavonoid content in tartary buckwheat. J Agric Food Chem 60:5161–5168
Li X, Thwe AA, Park NI, Suzuki T, Kim SJ, Park SU (2012b) Accumulation of phenylpropanoids and correlated gene expression during the development of tartary buckwheat sprouts. J Agric Food Chem 60:5629–5635
Liu B, Zhu Y (2007) Extraction of flavonoids from flavonoid-rich parts in tartary buckwheat and identification of the main flavonoids. J Food Eng 78:584–587
Mano H, Ogasawara F, Sato K, Higo H, Minobe Y (2007) Isolation of a regulatory gene of anthocyanin biosynthesis in tuberous roots of purple-fleshed sweet potato. Plant Physiol 143:1252–1268
Matsui K, Umemura Y, Ohme-Takagi M (2008) AtMYBL2, a protein with a single MYB domain, acts as a negative regulator of anthocyanin biosynthesis in Arabidopsis. Plant J 55:954–967
Nagata T, Todoriki S, Masumizu T, Suda I, Furuta S, Du Z, Kikuchi S (2003) Levels of active oxygen species are controlled by ascorbic acid and anthocyanin in Arabidopsis. J Agric Food Chem 51:2992–2999
Tsurunaga Y, Takahashi T, Katsube T, Kudo A, Kuramitsu O, Ishiwata M, Matsumoto S (2013) Effects of UV-B irradiation on the levels of anthocyanin, rutin and radical scavenging activity of buckwheat sprouts. Food Chem 141:552–556
Ubi BE, Honda C, Bessho H, Kondo S, Wada M, Kobayashi S, Moriguchi T (2006) Expression analysis of anthocyanin biosynthetic genes in apple skin: effect of UV-B and temperature. Plant Sci 170:571–578
Watanabe M (2007) An anthocyanin compound in buckwheat sprouts and its contribution to antioxidant capacity. Biosci Biotechnol Biochem 71:579–582
Xie X, Li S, Zhang R, Zhao J, Chen Y, Zhao Q, Yao Y, You C, Zhang X, Hao Y (2012) The bHLH transcription factor MdbHLH3 promotes anthocyanin accumulation and fruit colouration in response to low temperature in apples. Plant Cell Environ 35:1884–1897
Zhang Y, Zheng S, Liu Z, Wang L, Bi Y (2011) Both HY5 and HYH are necessary regulators for low temperature-induced anthocyanin accumulation in Arabidopsis seedlings. J Plant Physiol 168:367–374
Zhang B, Hu Z, Zhang Y, Li Y, Zhou S, Chen G (2012) A putative functional MYB transcription factor induced by low temperature regulates anthocyanin biosynthesis in purple kale (Brassica Oleracea var. acephala f. tricolor). Plant Cell Rep 31:281–289
Acknowledgments
Authors would like to thank Mogo Internet Technology Limited for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Gao.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11738_2015_1913_MOESM1_ESM.doc
Primers used for cDNAs cloning and semi-quantitative RT-PCR analyses are shown in Table S1; Partial sequences of isolation genes are shown in Fig. S2. Sequence multi-alignment of the deduced protein is shown in Fig. S3. (DOC 5823 kb)
Rights and permissions
About this article
Cite this article
Li, SJ., Bai, YC., Li, CL. et al. Anthocyanins accumulate in tartary buckwheat (Fagopyrum tataricum) sprout in response to cold stress. Acta Physiol Plant 37, 159 (2015). https://doi.org/10.1007/s11738-015-1913-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-015-1913-9