Abstract
The regulatory function of sucrose in the activity of lipid-degrading enzymes was investigated in germinating seeds of yellow lupin (Lupinus luteus L.), white lupin (Lupinus albus L.) and Andean lupin (Lupinus mutabilis Sweet). The study was conducted on isolated embryo axes, excised cotyledons and seedlings cultured in vitro for 96 h on medium with 60 mM sucrose or without the sugar. The activity of lipase (lipolysis), acyl-CoA oxidase and catalase (fatty acid β-oxidation) was enhanced in all studied organs cultured on medium without sucrose. The activity of cytosolic aconitase (glyoxylate cycle) was stimulated by sucrose in seedling axes and isolated embryo axes, whereas in seedling cotyledons and excised cotyledons, it was inhibited. The regulatory function of sucrose in phosphoenolpyruvate carboxykinase (gluconeogenesis) was observed only in isolated embryo axes and the activity was lower in carbohydrate deficiency conditions. The peculiar features of storage lipid breakdown in germinating lupin seeds and its regulation by sucrose are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lupin seeds differ significantly in storage lipid content. The clearest example of a high lipid level in mature seeds is Andean lupin (Lupinus mutabilis Sweet), which accumulates up to 20 % of lipid in seed dry matter. Opposite to Andean lupin is yellow lupin (Lupinus luteus L.). Lipid content in seeds of this species is about 6 %. White lupin seeds (Lupinus albus L.) contain 7–14 % (Borek et al. 2009, 2012a). Regarding storage lipid content, Andean lupin seeds are similar to soybean seeds, which contain 12–26 % of storage lipid (Zhou et al. 2006). Apart from storage lipid, lupin seeds contain large quantities of protein (up to 40–50 %), which is the dominant storage compound in these species (Santos et al. 1997; Duranti et al. 2008; Borek et al. 2012b).
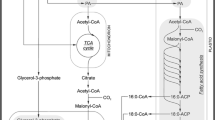
During seed germination, storage lipid is converted into sucrose (Penfield et al. 2005; Graham 2008), which is one of the main transport carbon forms in plants. This is a multi-step process involving several cell compartments (Fig. 1). Breakdown of storage lipid starts in oleosomes, where lipase liberates fatty acids from triacylglycerols (Graham 2008; Quettier and Eastmond 2009; Barros et al. 2010). Next, fatty acids such as acyl-CoA are oxidized in peroxisomes (sometimes called a glyoxysomes in oilseeds) through β-oxidation. This process is initiated by acyl-CoA oxidase. Fatty acid β-oxidation generates acetyl-CoA and additionally H2O2, which is detoxified by catalase (Baker et al. 2006; Graham 2008; Kaur et al. 2009; Contento and Bassham 2010). Acetyl-CoA enters the glyoxylate cycle that occurs both in the peroxisome and in the cytosol. The glyoxylate cycle is catalyzed by five enzymes, namely citrate synthase, aconitase, isocitrate lyase, malate synthase, and malate dehydrogenase. Two of them, i.e. aconitase and malate dehydrogenase, operate in the cytosol. Malate dehydrogenase occurs in the peroxisome as well but is not involved in the glyoxylate cycle (Pracharoenwattana et al. 2007; Pracharoenwattana and Smith 2008). Succinate arising in the peroxisome is transported into the mitochondrion and through part of the tricarboxylic acid (TCA) cycle is converted to malate. Subsequent steps occur in the cytosol, where malate is converted to oxaloacetate. In the reaction catalyzed by phosphoenolpyruvate carboxykinase, oxaloacetate is converted to phosphoenolpyruvate, which starts the gluconeogenesis pathway (Graham 2008). The above-mentioned process is well documented in oil-storing seeds but occurs in germinating lupin seeds as well (Borek et al. 2003, 2011; Borek and Ratajczak 2010). During lupin seed germination, not all storage lipid is converted to sucrose. Some of it is utilized as a respiratory substrate and is used for amino acid synthesis. It has been proven that lipid-derived carbon skeletons in germinating yellow lupin seeds are used for synthesis of, for example, asparagine, glutamate and glutamine. It is postulated that in yellow lupin, there exist several pathways leading from storage lipid to amino acids. Probably citrate and isocitrate (cytosolic aconitase substrate and product, respectively) are subtracted from the above-described pathway of storage lipid conversion to sucrose, and are used for amino acid synthesis (Fig. 1). In this process, cytosolic isocitrate dehydrogenase plays an important role (Borek et al. 2003; Borek and Ratajczak 2010). Further research has shown that the intensity of lipid breakdown and carbon flow from lipid to amino acids depends on the lipid content in lupin seeds. It has been proven by applying an inhibitor of asparagine synthesis (l-methionine sulfoximine—inhibitor of glutamine synthetase). When asparagine synthesis is disrupted, a slight increase in lipid breakdown is observed in yellow and white lupin embryo axes and cotyledons, whereas in Andean lupin embryo axes and cotyledons occurs the significant enhancement in lipid utilization (Borek et al. 2011).
Since sucrose is one of the main end products of storage lipid breakdown during seed germination and seedling establishment, it is considered as a regulatory agent. It is well documented that decreased sucrose or glucose levels in tissues enhance, for example, lipid (Dieuaide et al. 1992; Yu 1999; To et al. 2002; Borek et al. 2006, 2012a), protein (Brouquisse et al. 1991; Yu 1999; Borek and Ratajczak 2002; Borek et al. 2012b), and starch (Thomas and Rodriquez 1994; Borek et al. 2006, 2013) breakdown. In such conditions, photosynthesis is enhanced (Yu 1999) and autophagy may occur as well (Aubert et al. 1996; Yu 1999; Inoue and Moriyasu 2006; Bassham 2007). There are many data confirming that sucrose and glucose regulate plant metabolism by modulation of gene expression. In Arabidopsis thaliana seedlings, hundreds of genes are stimulated or repressed by glucose (Price et al. 2004). Studies conducted on germinating yellow lupin seeds have shown that sucrose regulates the activity of several enzymes involved in storage lipid breakdown and the changes in enzyme activities are a result of modification in gene expression. During yellow lupin seed germination, lipase and catalase activity increases in sugar deficiency conditions (Borek et al. 2006; Borek and Nuc 2011), and in the same conditions, the lipase mRNA level is higher as well (Borek and Nuc 2011). Contrary to lipase and catalase, other enzymes involved in further steps of lipid breakdown (cytosolic aconitase, isocitrate lyase, cytosolic isocitrate dehydrogenase) are less active in lupin embryo axes and cotyledons not fed with sucrose. Simultaneously, mRNA levels for cytosolic aconitase and cytosolic isocitrate dehydrogenase are lower (Borek and Nuc 2011). Higher activity and mRNA level for cytosolic aconitase and cytosolic isocitrate dehydrogenase in yellow lupin organs fed with sucrose are correlated with the enhanced carbon flow from lipid into amino acids, because amino acid synthesis from lipid is significantly enhanced by sucrose in embryo axes and cotyledons (Borek and Ratajczak 2010).
Till now, the regulatory function of sucrose in enzyme activities involved in storage lipid breakdown has been investigated only in yellow lupin seeds. This lupin species contains only about 6 % lipid in seeds. It is not known how sucrose regulates lipid-degrading enzymes in lupin species which accumulate more lipids in seeds. Therefore, this study was extended to white and Andean lupin seeds (lipid content 7–14 % and about 20 %, respectively) because, as mentioned above, the intensity of lipid breakdown and carbon flow from lipid to amino acids depends on lipid content in lupin seeds (Borek et al. 2011). To investigate the regulatory function of sucrose, the isolated embryo axes, excised cotyledons and seedlings were cultured in vitro on medium with 60 mM sucrose or without the sugar. Sucrose added to the medium in this concentration causes a significant increase in carbohydrate level in yellow lupin embryo axes, cotyledons and seedlings. Lack of sucrose in the medium causes a considerable decrease in sugar content in tissues, especially in isolated embryo axes (Borek et al. 2006). Experiments were conducted both on embryo axes and cotyledons because embryo axes contain less storage lipid than cotyledons (Borek et al. 2012a). In this study, the activities of lipase, acyl-CoA oxidase, catalase, cytosolic and mitochondrial aconitase, and phosphoenolpyruvate carboxykinase were assayed.
Materials and methods
Plant material
Yellow, white and Andean lupin seeds were surface-sterilized in 0.02 % HgCl2 for 10, 15, and 20 min (respectively) and allowed to imbibe in the dark for 24 h at 25 °C. Embryo axes and cotyledons isolated from imbibed seeds, as well as whole imbibed seeds deprived of their coats, were placed on sterilized filter paper (Whatman no. 3) in sterile tubes above Heller’s medium (Heller 1954) in two trophic variants: with 60 mM sucrose (+S) and without sucrose (−S). Isolated embryo axes, excised cotyledons and seedlings were cultured in vitro for 96 h in the dark at 25 °C. All experiments were conducted on isolated organs as well as on seedling organs because it enabled the detection of an undesirable effect of injury, i.e. isolation of organs.
Enzyme activity assays
Lipase (EC 3.1.1.3) activity was measured according to Marriott and Northcote (1975), described in detail by Borek et al. (2006). Enzyme activity was measured spectrophotometrically (Ultrospec 4000, Pharmacia Biotech) at λ = 420 nm using p-nitrophenol palmitate as substrate. Lipase activity was expressed in units (U) calculated on the basis of the increase in p-nitrophenol concentration read from a standard curve made for solutions ranging from 0.005 to 0.08 nM.
Acyl-CoA oxidase (EC 1.3.3.6) activity was measured according to Gerhard (1987). Enzyme activity was assayed by following H2O2 formation in a coupled assay which determines H2O2 in a peroxidatic reaction. Plant material was homogenized in 150 mM Tris–HCl buffer (pH 7.5), supplemented with 0.1 % 2-mercaptoethanol and 10 μM FAD. Buffer was used in the amount of 3 ml per 1 g of fresh weight. The homogenate was centrifuged for 20 min at 22,000g. The reaction mixture (1 ml) contained 150 mM Tris–HCl buffer (pH 7.5), 50 μM palmitoyl-CoA, 50 μM FAD, 13 mM 4-hydroxybenzoic acid, 1 mM aminoantipyrine, 1 mM NaN3, 5.3 U peroxidase, and 40 μl of supernatant. Absorbance was measured at λ = 500 nm for 15 min (Ultrospec 4000, Pharmacia Biotech). An increase in absorbance of about 0.51 was equal to decomposition of 0.1 μmol ml−1 H2O2.
Catalase (EC 1.11.1.6) activity was measured according to Lück (1965), described in detail by Borek et al. (2006). Enzyme activity was measured spectrophotometrically (Ultrospec 4000, Pharmacia Biotech) at λ = 240 nm using H2O2 as substrate (molar absorption coefficient for H2O2 = 43.6).
Aconitase (EC 4.2.1.3) activity was measured in cytosolic and mitochondrial fractions according to Kennedy et al. (1983) and was described in detail by Borek and Nuc (2011). To assess the quality of the cytosolic fraction, glutamate dehydrogenase activity (marker of mitochondria) was simultaneously assayed (Borek et al. 2012b). Enzyme activity was measured spectrophotometrically (Ultrospec 4000, Pharmacia Biotech) at λ = 240 nm using sodium isocitrate as the substrate. The increase in cis-aconitate was measured.
Phosphoenolpyruvate carboxykinase (EC 4.1.1.49) activity was measured according to Chen et al. (2004). Enzyme activity was measured in the carboxylation direction, in which oxaloacetate formed is reduced to malate by malate dehydrogenase and NADH. Plant material was homogenized in 10 mM HEPES–KOH buffer (pH 7.0) supplemented with 0.1 % 2-mercaptoethanol. Buffer was used in the amount of 3 ml per 1 g of fresh weight. The homogenate was centrifuged for 20 min at 22,000g. The reaction mixture (1 ml) contained 100 mM HEPES–KOH buffer (pH 7.0), 6 mM phosphoenolpyruvate, 90 mM KHCO3, 2 mM ADP, 100 mM KCl, 0.1 % 2-mercaptoethanol, 6 mM MgSO4·7H2O, 0.3 mM NADH, 6 U malate dehydrogenase, and 50 μl of supernatant. Absorption was measured at λ = 340 nm for 15 min (Ultrospec 4000, Pharmacia Biotech).
Protein determination
Protein concentration in enzyme extracts was determined according to Bradford’s (1976) method, with BSA as a standard.
Statistical analysis
The results are the mean ± SD of three independent experiments with two or three replications each. Significance of differences between mean values was determined with Student’s t test. The statistical analysis was connected only to assessing the significance in the differences between organs cultured in vitro on medium containing 60 mM sucrose (+S) and organs cultured on medium without sucrose (−S).
Results
Activity of the enzymes investigated was differently regulated by sucrose. Lipase (Fig. 2), acyl-CoA oxidase (Fig. 3) and catalase (Fig. 4) activity was enhanced in all studied organs cultured on medium without sucrose (−S). The regulatory function of sucrose on aconitase activity was different in axes and cotyledons (Fig. 5). In Andean lupin seedling axes and isolated embryo axes, the activity of cytosolic and mitochondrial aconitase was higher in sucrose supply conditions (+S), whereas in seedling cotyledons and excised cotyledons, aconitase (both cytosolic and mitochondrial) activity was lower in organs cultured on medium +S (Fig. 5). To assess the purity of cytosolic and mitochondrial fractions, the activity of glutamate dehydrogenase (a mitochondrial marker) was simultaneously assayed. Glutamate dehydrogenase activity in the cytosolic fraction was negligible compared to its mitochondrial activity, and was described in detail in another paper (Borek et al. 2012b). The distinct effect of in vitro culture trophic conditions on phosphoenolpyruvate carboxykinase activity was detected only in isolated embryo axes of all three investigated lupin species. In these organs, the enzyme activity was significantly decreased in carbohydrate depletion conditions (−S; Fig. 6).
Discussion
The regulatory function of sucrose in activity of enzymes involved in storage lipid breakdown during seed germination of yellow, white and Andean lupin was investigated. Enzymes which take part in the first steps of storage lipid breakdown, i.e. lipase (lipolysis) as well as acyl-CoA oxidase and catalase (fatty acid β-oxidation), were significantly more active in organs cultured on medium without sucrose (−S; Figs. 2, 3, 4, respectively; activity of lipase and catalase in organs of yellow lupin was described earlier by Borek et al. 2006). Catalase is associated with fatty acid β-oxidation and a rise in its activity can be treated as an indirect proof of intensification of the fatty acid β-oxidation in sugar-starved tissues (Dieuaide et al. 1992, 1993; Contento and Bassham 2010). Such an increase in enzyme activity is consistent with many literature data describing intensification of processes leading to supply of respiratory substrates in carbohydrate depletion conditions (Brouquisse et al. 1991; Yu 1999; Borek et al. 2001; Gonzali et al. 2006). It is highly possible that increased activity of the abovementioned enzymes in sugar deficiency conditions was regulated upon the mechanism of so-called catabolic repression. The catabolic repression mechanism was earlier described in detail in bacteria and yeast (Saier 1989; Gancedo 1992). In higher plants, it is involved in the more general system of gene expression control by metabolic signals and plays an important role in metabolic regulation, particularly in tissues and organs that are not capable of photosynthesis (Koch 1996; Smeekens and Rook 1997; Brouquisse et al. 1998). In carbohydrate-starved tissues, the expression of many genes is enhanced (Koch 1996; Gonzali et al. 2006; Rolland et al. 2006; Li et al. 2006; Ramon et al. 2008; Smeekens et al. 2010) and it leads to increased activity of relevant enzymes. Such enhanced gene expression for lipase was described in yellow lupin axes and cotyledons in which the sugar level was decreased (Borek and Nuc 2011). In carbohydrate-starved cucumber cell culture, an increase in mRNA for malate synthase and isocitrate lyase (two enzymes of the glyoxylate cycle; Graham et al. 1994) was detected. In this study, changes in mRNA levels were not determined but the increase in lipase, acyl-CoA oxidase and catalase activity (Figs. 2, 3, 4, respectively) was probably a result of stimulated gene expression in sugar deficiency conditions.
In germinating yellow lupin seeds, a strong relationship between storage lipid breakdown and amino acid synthesis was discovered (Fig. 1). It has been evidenced that lipid-derived carbon skeletons were used for synthesis among others of asparagine, glutamine and glutamate. In seedling axes, sucrose (+S) enhanced the carbon flow from lipid into amino acids but in seedling cotyledons, the carbon flow was higher in −S conditions. Contrary to this, in isolated embryo axes and cotyledons cultured in vitro, the carbon flow was more intense in sucrose-fed conditions (+S) (Borek et al. 2003; Borek and Ratajczak 2010). One of the key enzymes involved in pathways of carbon flow from lipids into amino acids is cytosolic aconitase (Fig. 1; Borek and Ratajczak 2010; Borek et al. 2011). Mitochondrial aconitase operates in the TCA cycle. Cytosolic and mitochondrial aconitase activity and its regulation by sucrose in yellow lupin germinating seeds was described earlier (Borek and Nuc 2011). In this paper, the activity of aconitase in Andean lupin organs is presented (Fig. 4). It is highly possible that in seeds of this lupin species, there also exists carbon flow from lipid into amino acids. This conclusion is based on the following: (1) Andean lupin seeds contain much more storage lipid than yellow lupin seeds (Borek et al. 2009, 2012a) and more carbon skeletons might be used for amino acid synthesis; (2) disruption in asparagine synthesis caused a significant increase in fatty acid utilization (Borek et al. 2011); (3) the changes caused by sucrose in aconitase activity (Fig. 5) were very similar to those observed in aconitase activity in yellow lupin seeds (Borek and Nuc 2011); and (4) the changes caused by sucrose in aconitase activity (Fig. 5) correlate with the changes caused by sucrose in carbon flow from lipid into amino acids in yellow lupin (Borek et al. 2003; Borek and Ratajczak 2010).
Phosphoenolpyruvate carboxykinase is one of the enzymes involved in the pathway of commonly known conversion of storage lipid into sugars during seed germination. It is active in the cytosol and takes part in gluconeogenesis (Graham 2008). Changes caused by sucrose in phosphoenolpyruvate carboxykinase activity in germinating lupin seeds were significant only in isolated embryo axes of three investigated lupin species. In sucrose-starved (−S) isolated embryo axes, the activity was significantly lower than in the sucrose-fed (+S) ones (Fig. 6). An explanation of this result is possible on the basis of our previously performed experiments with radiolabeled acetate (the simplest fatty acid) and upon analysis of incorporation of acetate-derived carbons into sugars (Borek et al. 2003; Borek and Ratajczak 2010). In yellow lupin seeds, conversion of lipid into sugars is considerably retarded in carbohydrate-depletion conditions. The highest restriction of sugar synthesis from lipids was observed in isolated embryo axes. When the carbohydrate level in tissues was decreased, the intermediates of storage lipid breakdown (for example intermediates of glyoxylate cycle) might be used as respiratory substrates and cannot be used for sugar synthesis (Borek et al. 2003; Borek and Ratajczak 2010).
Summarizing the data presented in this paper, it can be concluded that sucrose is a very important regulatory agent in activity of enzymes taking part in storage lipid breakdown in yellow, white and Andean lupin-germinating seeds. A decreasing carbohydrate level in tissues caused diverse changes in enzymes activity. Some enzymes, such as lipase, acyl-CoA oxidase and catalase, were stimulated in those conditions in all organs investigated, whereas others, for example phosphoenolpyruvate carboxykinase in isolated embryo axes, were less active. The activity of aconitase was differently regulated in axes and cotyledons. This special feature of the sucrose regulatory function occurred in all three lupin species. It was very similar in organs of all three investigated lupine species and was independent of the amount of storage lipid in seeds.
Author contribution
Sławomir Borek designed the experiment, assayed activity of acyl-CoA oxidase and phosphoenolpyruvate carboxykinase, analyzed results, and wrote this manuscript. Szymon Kubala assayed activity of aconitase. Sylwia Kubala assayed activity of lipase and catalase.
References
Aubert S, Gout E, Bligny R, Marty-Mazars D, Barrieu F, Alabouvette J, Marty F, Douce R (1996) Ultrastructural and biochemical characterization of autophagy in higher plant cells subjected to carbon deprivation: control by the supply of mitochondria with respiratory substrates. J Cell Biol 133:1251–1263
Baker A, Graham IA, Holdsworth M, Smith SM, Theodoulou FL (2006) Chewing the fat: β-oxidation in signalling and development. Trends Plant Sci 11:124–132. doi:10.1016/j.tplants.2006.01.005
Barros M, Fleuri LF, Macedo GA (2010) Seed lipases: sources, applications and properties—a review. Braz J Chem Eng 27:15–29. doi:10.1590/S0104-66322010000100002
Bassham DC (2007) Plant autophagy—more than a starvation response. Curr Opin Plant Biol 10:587–593. doi:10.1016/j.pbi.2007.06.006
Borek S, Nuc K (2011) Sucrose controls storage lipid breakdown on gene expression level in germinating yellow lupine (Lupinus luteus L.) seeds. J Plant Physiol 168:1795–1803. doi:10.1016/j.jplph.2011.05.016
Borek S, Ratajczak W (2002) Sugars as a metabolic regulator of storage protein mobilization in germinating seeds of yellow lupine (Lupinus luteus L.). Acta Physiol Plant 24:425–434. doi:10.1007/s11738-002-0039-z
Borek S, Ratajczak L (2010) Storage lipids as a source of carbon skeletons for asparagine synthesis in germinating seeds of yellow lupine (Lupinus luteus L.). J Plant Physiol 167:717–724. doi:10.1016/j.jplph.2009.12.010
Borek S, Morkunas I, Ratajczak W, Ratajczak L (2001) Metabolism of amino acids in germinating yellow lupine seeds III. Breakdown of arginine in sugar-starved organs cultivated in vitro. Acta Physiol Plant 23:141–148. doi:10.1007/s11738-001-0001-5
Borek S, Ratajczak W, Ratajczak L (2003) A transfer of carbon atoms from fatty acids to sugars and amino acids in yellow lupine (Lupinus luteus L.) seedlings. J Plant Physiol 160:539–545. doi:10.1078/0176-1617-00763
Borek S, Ratajczak W, Ratajczak L (2006) Ultrastructural and enzymatic research on the role of sucrose in mobilization of storage lipids in germinating yellow lupine seeds. Plant Sci 170:441–452. doi:10.1016/j.plantsci.2005.09.011
Borek S, Pukacka S, Michalski K, Ratajczak L (2009) Lipid and protein accumulation in developing seeds of three lupine species: Lupinus luteus L., Lupinus albus L., and Lupinus mutabilis Sweet. J Exp Bot 60:3453–3466. doi:10.1093/jxb/erp186
Borek S, Kubala S, Kubala S, Ratajczak L (2011) Comparative study of storage compound breakdown in germinating seeds of three lupine species. Acta Physiol Plant 33:1953–1968. doi:10.1007/s11738-011-0744-6
Borek S, Kubala S, Kubala S (2012a) Regulation by sucrose of storage compounds breakdown in germinating seeds of yellow lupine (Lupinus luteus L.), white lupine (Lupinus albus L.) and Andean lupine (Lupinus mutabilis Sweet). I. Mobilization of storage protein. Acta Physiol Plant 34:701–711. doi:10.1007/s11738-011-0870-1
Borek S, Pukacka S, Michalski K (2012b) Regulation by sucrose of storage compounds breakdown in germinating seeds of yellow lupine (Lupinus luteus L.), white lupine (Lupinus albus L.) and Andean lupine (Lupinus mutabilis Sweet). II. Mobilization of storage lipid. Acta Physiol Plant 34:1199–1206. doi:10.1007/s11738-011-0916-4
Borek S, Galor A, Paluch E (2013) Asparagine enhances starch accumulation in developing and germinating lupin seeds. J Plant Growth Reg. doi:10.1007/s00344-012-9313-5
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Brouquisse R, James F, Rajmond P, Pradet A (1991) Study of glucose starvation in excised maize root tips. Plant Physiol 96:619–626. doi:10.1104/pp.96.2.619
Brouquisse R, Gaudillere JP, Raymond P (1998) Induction of a carbon-starvation-related proteolysis in whole maize plants submitted to light/dark cycles and to extended darkness. Plant Physiol 117:1281–1291. doi:10.1104/pp.117.4.1281
Chen ZH, Walker RP, Técsi LI, Lea PJ, Leegood RC (2004) Phosphoenolpyruvate carboxykinase in cucumber plants is increased both by ammonium and by acidification, and is present in phloem. Planta 219:48–58. doi:10.1007/s00425-004-1220-y
Contento AL, Bassham DC (2010) Increase in catalase-3 activity as a response to use of alternative catabolic substrates during sucrose starvation. Plant Physiol Biochem 48:232–238. doi:10.1016/j.plaphy.2010.01.004
Dieuaide M, Brouquisse R, Pradet A, Raymond P (1992) Increased fatty acid β-oxidation after glucose starvation in maize root tips. Plant Physiol 99:595–600. doi:10.1104/pp.99.2.595
Dieuaide M, Couée I, Pradet A, Raymond P (1993) Effects of glucose starvation on the oxidation of fatty acids by maize root tip mitochondria and peroxisomes: evidence for mitochondrial fatty acid β-oxidation and acyl-CoA dehydrogenase activity in higher plant. Biochem J 296:199–207
Duranti M, Consonni A, Magni C, Sessa F, Scarafoni A (2008) The major proteins of lupin seed: Characterisation and molecular properties for use as functional and nutraceutical ingredients. Trends Food Sci Tech 19:624–633. doi:10.1016/j.tifs.2008.07.002
Gancedo J (1992) Carbon catabolite repression in yeast. Eur J Biochem 206:297–313
Gerhard B (1987) Peroxisome and fatty acid degradation. Methods Enzymol 148:516–525
Gonzali S, Loreti E, Solfanelli C, Novi G, Alpi A, Perata P (2006) Identification of sugar-modulated genes and evidence for in vivo sugar sensing in Arabidopsis. J Plant Res 119:115–123. doi:10.1007/s10265-005-0251-1
Graham IA (2008) Seed storage oil mobilization. Annu Rev Plant Biol 59:115–142. doi:10.1146/annurev.arplant.59.032607.092938
Graham IA, Derby KJ, Leaver CJ (1994) Carbon catabolite repression regulates glyoxylate cycle gene expression in cucumber. Plant Cell 6:761–772. doi:10.1105/tpc.6.5.761
Heller R (1954) Recherches sur la nutrition minérale des tissus végétaux ciltivés in vitro. Annu Sci Nat Bot Biol Veg 14:1–223
Inoue Y, Moriyasu Y (2006) Autophagy is not a main contributor to the degradation of phospholipids in tobacco cells cultured under sucrose starvation conditions. Plant Cell Physiol 47:471–480. doi:10.1093/pcp/pcj013
Kaur N, Reumann S, Hu J (2009) Peroxisome biogenesis and function. The Arabidopsis Book, American Society of Plant Biologist. http://www.bioone.org/doi/abs/10.1199/tab.0123. Accessed 11 September 2009
Kennedy MC, Emptage MH, Dreyer JL, Beinert H (1983) The role of iron in the activation-inactivation of aconitase. J Biol Chem 258:11098–11105
Koch KE (1996) Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol 47:509–540. doi:10.1146/annurev.arplant.47.1.509
Li Y, Lee KK, Walsh S, Smith C, Hadingham S, Sorefan K, Cawley G, Bevan MW (2006) Establishing glucose- and ABA-regulated transcription networks in Arabidopsis by microarray analysis and promoter classification using a relevance vector machine. Genome Res 16:414–427. doi:10.1101/gr.4237406
Lück H (1965) Catalase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Academic Press, New York, pp 885–888
Marriott KM, Northcote DH (1975) The induction of enzyme activity in the endosperm of germinating castor bean. Biochem J 152:65–70
Penfield S, Graham I, Graham IA (2005) Storage reserve mobilization in germinating oilseeds: Arabidopsis as a model system. Biochem Soc Trans 33:380–383
Pracharoenwattana I, Smith SM (2008) When is a peroxisome not a peroxisome? Trends Plant Sci 13:522–525. doi:10.1016/j.tplants.2008.07.003
Pracharoenwattana I, Cornah JE, Smith SM (2007) Arabidopsis peroxisomal malate dehydrogenase functions in β-oxidation but not in the glyoxylate cycle. Plant J 50:381–390. doi:10.1111/j.1365-313X.2007.03055.x
Price J, Laxmi A, Martin SKST, Jang JC (2004) Global transcription profiling reveals multiple sugar signal transduction mechanisms in Arabidopsis. Plant Cell 16:2128–2150. doi:10.1105/tpc.104.022616
Quettier AL, Eastmond PJ (2009) Storage oil hydrolysis during early seedling growth. Plant Physiol Biochem 47:485–490. doi:10.1016/j.plaphy.2008.12.005
Ramon M, Rolland F, Sheen J (2008) Sugar sensing and signaling. The Arabidopsis Book, American Society of Plant Biologists. http://www.bioone.org/doi/abs/10.1199/tab.0117. Accessed 22 October 2008
Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57:675–709. doi:10.1146/annurev.arplant.57.032905.105441
Saier M (1989) Protein phosphorylation and allosteric control of inducer exclusion and catabolite repression by the bacterial phosphoenolpyruvate: sugar phosphotransferase system. Microbiol Rev 53:109–120
Santos CN, Ferreira RB, Teixeira AR (1997) Seeds proteins of Lupinus mutabilis. J Agric Food Chem 45:3821–3825. doi:10.1021/jf970075v
Smeekens S, Rook F (1997) Sugar sensing and sugar-mediated signal transduction in plants. Plant Physiol 115:7–13. doi:10.1104/pp.115.1.7
Smeekens S, Jingkun M, Johannes H, Rolland F (2010) Sugar signals and molecular networks controlling plant growth. Curr Opin Plant Biol 13:274–279. doi:10.1016/j.pbi.2009.12.002
Thomas BR, Rodriquez R (1994) Metabolite signals regulate gene expression and source/sink relations in cereal seedlings. Plant Physiol 106:1235–1239. doi:10.1104/pp.106.4.1235
To JPC, Reiter WD, Gibson SI (2002) Mobilization of seed storage lipid by Arabidopsis seedlings is retarded in the presence of exogenous sugars. BMC Plant Biol 2:4. doi:10.1186/1471-2229-2-4
Yu SM (1999) Cellular and genetic responses of plants to sugar starvation. Plant Physiol 121:687–693. doi:10.1104/pp.121.3.687
Zhou S, Zhang D, Luan H, Yu F, Xin X, Hu G (2006) Primary study on protein and lipid accumulation in high oil content soybean varieties. Chin J Oil Crop Sci 28:214–216
Acknowledgments
We thank Dr. Stanisław Stawinski, Head of Plant Breeding Station Smolice Division in Przebędowo (Murowana Goślina, Poland), for providing lupin seeds. This work was partially supported by grant no. 2 P06A 004 29 from Ministry of Science and Higher Education.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. van Staden.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Borek, S., Kubala, S. & Kubala, S. Diverse regulation by sucrose of enzymes involved in storage lipid breakdown in germinating lupin seeds. Acta Physiol Plant 35, 2147–2156 (2013). https://doi.org/10.1007/s11738-013-1251-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-013-1251-8