Abstract
Research of the regulatory function of sucrose in storage protein breakdown was conducted on isolated embryo axes, excised cotyledons and whole seedlings of three lupine species grown in vitro on medium with 60 mM sucrose or without the sugar. Sucrose stimulated growth of yellow, white and Andean lupine isolated embryo axes and cotyledons but growth of seedlings was inhibited. Dry matter content was higher in sucrose-fed isolated organs and in seedling organs. Ultrastructure research revealed that lack of sucrose in the medium caused enhancement in storage protein breakdown. Protein deposits in cotyledons were smaller as well as soluble portion content in all studied organs was lower when there was no sucrose in the medium. In the same conditions, the activity of glutamate dehydrogenase was significantly higher. Increase in vacuolization of cells of white lupine root meristematic zone cells was observed and induction of autophagy in young carbohydrate-starved embryo axes is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Protein is the main storage compound in lupine seeds. Yellow lupine seeds contain about 45% (Cerletti 1982), white lupine up to 38% (Mohamed and Rayas-Duarte 1995) and Andean lupine 40–50% (Santos et al. 1997) of dry matter. Lupine seeds contain two classes of proteins which, according to Osborn’s classification, correspond to the albumin and globulin fractions (Duranti et al. 2008). Globulins dominate in lupine seeds. In yellow lupine they might constitute up to 80% of the total seed protein content (Gwóźdź 1988; Ratajczak et al. 1999). In white lupine seeds, albumins to globulins ratio is about 1/9 (Duranti et al. 2008) and in Andean lupine seeds this ratio is about 1/5 (Santos et al. 1997). Lupine seeds considerably differ among species upon storage lipid content. Seeds of yellow lupine contain about 6%, yellow lupine 7–14%, and in Andean lupine seeds lipid content might reach about 20% of dry matter (Borek et al. 2009). Mature lupine seeds contain no starch (Borek et al. 2006, 2011a; Duranti et al. 2008) and carbohydrates occur mainly as fiber (Hedley 2001).
In lupine seeds, storage protein is located in vacuoles of cotyledons (Borek and Ratajczak 2002; Borek et al. 2006). During seeds germination, storage protein serves as nitrogen and carbon skeleton sources for developing seedling. Large amount of amino acids liberated from storage protein is utilised in respiration (Ratajczak et al. 1996; Borek et al. 2001; Lehmann and Ratajczak 2008) or as glutamine and asparagine are translocated into growing axes (Bewley and Black 1994). One of the most abundant amino acid in yellow lupine storage protein is glutamate the level of which reaches 20–25% of amino acids content (Lehmann and Ratajczak 2008). Glutamate is involved in GS/GOGAT cycle (glutamine synthetase/glutamate synthase) in cytosol or is oxidized by glutamate dehydrogenase in mitochondria. Glutamate dehydrogenase oxidizes glutamate into 2-oxoglutarate and ammonium. 2-oxoglutarate might be utilised in respiration in TCA cycle and ammonium is translocated into cytosol where it is stored in asparagine trough the cooperative action of glutamine synthetase and asparagine synthase (Lehmann and Ratajczak 2008; Borek et al. 2011a). During yellow lupine seeds germination, high accumulation of asparagine is observed. Asparagine content might reach up to 30% of the dry matter of seedling embryo axes. Such high asparagine accumulation is a result of detoxification of ammonium produced by glutamate dehydrogenase. Ammonium might be produced as well by urease which catalyzes decomposition of urea produced during arginine breakdown (Borek et al. 2001) but arginine makes up only 6–10% of amino acid content of yellow lupine storage protein (Lehmann and Ratajczak 2008). The crucial role of glutamate dehydrogenase in glutamate utilization (catabolic action of enzyme) and amino acid conversions in germinating seeds of yellow lupine was described in detail by Lehmann and Ratajczak (2008). Nevertheless, glutamate dehydrogenase may also function anabolically in the direction of glutamate biosynthesis (from 2-oxoglutarate and ammonium). The catabolic and anabolic direction of the enzyme action is considered as an alternative and depends on carbon and ammonium conditions (Lehmann et al. 2011).
During lupine seeds germination breakdown of storage protein might be controlled by carbohydrate level in tissues. Up to now, research of the regulatory function of sugars in storage protein breakdown was conducted nearly exclusively on yellow lupine and sucrose was taken under consideration. Ultrastructural research of yellow lupine germinating seeds showed that deposits of storage protein were faster degraded in carbohydrate deficient conditions (Borek and Ratajczak 2002; Borek et al. 2006) and the significant increase in proteolytic activity was observed. Enhancement in activity of exo- and endopeptidases was noted already after 24 h of germination and was intensified in successive days of growth (Borek and Ratajczak 2002). Amino acids (including glutamate) derived from storage protein breakdown were used as respiratory substrates and utilization of amino acids was considerably enhanced in sugar depletion conditions (Borek et al. 2001; Lehmann et al. 2003). Activity (both catabolic and anabolic) of glutamate dehydrogenase was higher in sucrose-starved organs of yellow lupine seeds (Lehmann et al. 2003, 2010; Borek and Nuc 2011). It is strongly recommended that increase in glutamate dehydrogenase activity in carbohydrate depletion conditions in yellow lupine is caused by enhanced gene expression. Such interpretation is based on changes in the isoenzymatic pattern of glutamate dehydrogenase in yellow lupine embryo axes and on the results of experiments using antibodies and inhibitors of RNA and protein synthesis (Lehmann et al. 2003, 2010).
In the experiments described in this paper, seeds of three lupine species were used. Lupine species were chosen in such way that seeds chemical composition was clearly diversified. Especially seeds of Andean lupine are interesting because of simultaneous high content of storage protein (40–50%) and storage lipid (about 20% of the seeds dry matter). In germinating yellow lupine seeds which contain only 6% of lipid, strong connections between pathways of storage protein and storage lipid breakdown occurs. During germination storage lipid is used as respiratory substrate, but part of lipid-derived carbon skeletons is used for amino acids biosynthesis. Asparagine, glutamine and glutamate are the main amino acids synthesized from lipid-derived carbon skeletons (Borek et al. 2003; Borek and Ratajczak 2010). Moreover, when synthesis of asparagine is disrupted (by l-methionine sulfoximine—an inhibitor of glutamine synthetase), the increase in lipid utilization is observed. This increase has been noted in yellow, white and Andean lupine embryo axes and cotyledons but the most evident effect of asparagine synthesis inhibitor has been detected in organs of Andean lupine, i.e. lupine species seeds contain the highest amount of storage lipid (Borek et al. 2011a). So, the main aim of the research described in this paper was to check how sucrose regulates storage protein breakdown during lupine seeds germination and to check whether any differences in sucrose regulation exist among this three lupine species. To define the regulatory function of sucrose in storage protein breakdown the isolated embryo axes, excised cotyledons and seedlings of yellow, white and Andean lupine were grown in vitro on medium with 60 mM sucrose or without the sugar. Sucrose in this concentration added to the medium causes significant increase in soluble carbohydrate level in growing in vitro lupine seed organs (Borek et al. 2006). Morphology and ultrastructure were described, soluble protein content was determined and activity of glutamate dehydrogenase was assayed.
Materials and methods
Plant material
Yellow, white and Andean lupine seeds were surface-sterilized in 0.02% HgCl2 for 10, 15 and 20 min (respectively) and allowed to imbibe for 24 h at 25°C. Embryo axes and cotyledons isolated from imbibed seeds, as well as whole imbibed seeds deprived of their coats, were placed on sterilized filter paper (Whatman no. 3) in sterile tubes above Heller’s medium (Heller 1954) in two trophic variants: with 60 mM sucrose (+S) and without sucrose (−S). Isolated embryo axes, excised cotyledons and seedlings were cultured in vitro for 96 h in the dark at 25°C. Experiments were conducted on isolated organs as well as on seedling organs because it enabled to detect undesirable effect of injury i.e. isolation of organs.
Dry matter determination
Embryo axes and cotyledons of air dry and imbibed (24 h) yellow, white and Andean lupine seeds as well as organs grown in vitro for 96 h were dried in 105°C up to constant mass (2 h for isolated embryo axes, 2.5 h for seedling axes and 3 h for cotyledons). Dry matter (DM) was expressed as percent of fresh weight (FW).
Preparation of tissues for transmission electron microscopy (TEM)
Samples of white and Andean lupine seedling tissues (parenchyma cells of cotyledon, cortical parenchyma cells of hypocotyl and root tip) were fixed in a mixture of 2% glutaraldehyde and 2% paraformaldehyde (Karnovsky 1965). Postfixation was conducted in 1% OsO4. The samples were stained in 2% aqueous solution of uranyl acetate. Dehydration was performed in a series of acetone solutions. The samples were embedded in epoxy resin of low viscosity (Spurr 1969). Ultrathin sections were prepared using Ultracut S Raichert, stained in 5% uranyl acetate and 0.5% lead citrate and observed under the transmission electron microscope TEM-1200 Ex JEOL.
Soluble protein determination
Embryo axes and cotyledons of air dried and imbibed (24 h) yellow, white and lupine seeds as well as organs grown in vitro for 96 h were homogenized in 100 mM Tris–HCl buffer, pH 7.0 (3 ml per 1 g of fresh weight). The homogenate was centrifuged for 20 min at 22,000×g. Protein concentration in the supernatant was determined according to Bradford (1976), with bovine serum albumin used as a standard.
Glutamate dehydrogenase activity assay
The activity of glutamate dehydrogenase (NADH-GDH, EC 1.4.1.2) was assayed according to Watanabe et al. (1999) in cytosolic and mitochondrial fraction of Andean lupine isolated embryo axes, excised cotyledons and seedling axes and cotyledons grown in vitro for 96 h. The plant material was homogenized in 50 mM phosphate buffer (pH 7.5), supplemented with 300 mM mannitol and 1 mM EDTA. 2 ml of buffer were used for 1 g of fresh weight. Differential centrifugation was used to separate the cytosolic and mitochondrial fractions. The mitochondrial suspension was collected with 0.1% Triton X-100. The reaction mixture (1.5 ml) contained 100 mM of Tris–HCl buffer (pH 8.0), 10 mM 2-oxoglutarate, 100 mM NH4Cl, 65 mM NADH, 30 mM CaCl2, and 50 μl of cytosolic or mitochondrial fraction. Absorbance was measured at λ = 340 nm for 3 min in the spectrophotometer Ultrospec 4000 (Pharmacia Biotech).
Statistical analysis
The results are the mean ± SD of three independent experiments each with two or three replications. Significance of differences between mean values was determined with Student’s t test. The statistical analysis was connected only to assess the significance in the differences between organs grown in vitro on medium containing 60 mM sucrose (+S) and organs grown on medium without sucrose (−S).
Results
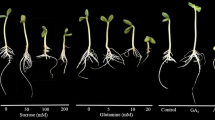
Yellow, white and Andean lupine seedlings grown for 96 h on medium with 60 mM sucrose (+S) were clearly smaller than those grown on medium without sucrose (−S; Figs. 1a, 2a, 3a, respectively). They were significantly lighter too (FW; Table 1). Contrary to this, isolated embryo axes grown on +S medium were considerably longer (Figs. 1b, 2b, 3b) and heavier (Table 1) than those sucrose-starved (−S). Excised cotyledons did not exhibit clear morphological changes between +S and −S variant (Figs. 1c, 2c, 3c); however, those fed with sucrose were heavier than those grown on −S medium (Table 1). Dry matter (DM) of seedling axes and cotyledons, isolated embryo axes and excised cotyledons of three studied lupine species was higher in organs grown on +S than −S medium (Table 1).
Morphology of yellow lupine seedlings (a), isolated embryo axes (b) and excised cotyledons (c) grown in vitro for 96 h on medium with 60 mM sucrose (+S) or without the sugar (−S). X, imbibed seeds (24 h) deprived of their coats (a) and isolated from imbibed seeds axes (b) and cotyledons (c); plant material used for preparing of in vitro culture. White bar 1 cm
Morphology of white lupine seedlings (a), isolated embryo axes (b) and excised cotyledons (c) grown in vitro for 96 h on medium with 60 mM sucrose (+S) or without the sugar (−S). X, imbibed seeds (24 h) deprived of their coats (a) and isolated from imbibed seeds axes (b) and cotyledons (c); plant material used for preparing of in vitro culture. White bar 1 cm
Morphology of Andean lupine seedlings (a), isolated embryo axes (b) and excised cotyledons (c) grown in vitro for 96 h on medium with 60 mM sucrose (+S) or without the sugar (−S). X, imbibed seeds (24 h) deprived of their coats (a) and isolated from imbibed seeds axes (b) and cotyledons (c); plant material used for preparing of in vitro culture. White bar 1 cm
The effect of sucrose on ultrastructure of yellow lupine seedlings was previously described in detail by Borek et al. (2006). Changes caused by sucrose in ultrastructure of isolated embryo axes and excised cotyledons of three investigated lupine species were described previously as well (Borek et al. 2011a). So in this paper, ultrastructure of only white and Andean lupine seedlings is presented. White lupine seedlings fed with sucrose (+S) contained in cotyledons much more storage protein than those grown on −S medium. It was reflected in almost fully filled vacuoles with protein deposits. In −S cotyledons, storage protein deposits were considerably smaller and their breakdown was clearly advanced (Fig. 4a, b). In +S cotyledons, oil bodies were larger and clearly more numerous. Similarly, starch granules were larger and more numerous (Fig. 4a, b). Moreover, cell wall was slightly ticker in +S cotyledons. Differences in cells of hypocotyl were mainly limited to appearance of many plasma membrane round structures inside the large central vacuole in −S organ (Fig. 4c, d) and to decreasing cell wall thickness (Fig. 4c, d). In hypocotyl cells, there were no deposits of storage protein, oil bodies and starch granules. In the cells of root meristematic zone, the deposits of storage protein were not visible but oil bodies and starch granules were present (Fig. 4e, f). Similar to cotyledon cells, oil bodies and starch granules were larger and more numerous in +S seedlings (Fig. 4c, d). In cells of this zone, in −S seedlings a significant increase in vacuolization level was observed (Fig. 4e, f). In root cap cells, large, numerous starch granules (Fig. 4g, h) and small, few bodies were visible. In root cap of +S seedlings, large quantities of mucilage (clearly fewer in −S) were noted (Fig. 4g, h). Changes caused by sucrose in Andean lupine seedlings were similar to those observed in white lupine seedlings. Larger deposits of storage protein were observed in cotyledons of +S seedlings (Fig. 5a, b). In +S cotyledons, oil bodies were larger and clearly more numerous (Fig. 5a, b). In addition, observations of ultrastructure of cotyledons showed distinctive differences in oil content in white and Andean lupine seeds. Considerably, more oil bodies were in the cotyledons of Andean lupine than in those of white lupine seedlings (compare Figs. 4a, 5a). Starch granules were small and not numerous (Fig. 5a), and no differences were observed in their size and number between +S and −S seedlings. Cell wall was slightly thicker in +S cotyledons. In the cells of Andean lupine +S seedling hypocotyls, there were no deposits of storage protein (Fig. 5c), but, contrary to the hypocotyls of white lupine, few oil bodies (Fig. 5c) and small starch granules were visible. In −S hypocotyls, oil bodies disappeared almost completely (Fig. 5d), and starch granules were smaller and less numerous. In the cells of root meristematic zone of +S seedlings, small deposits of storage protein, starch granules and oil bodies were observed (Fig. 5e). In the cells of the same zone of −S seedlings, storage protein and starch granules disappeared (Fig. 5f), and a slight decrease in the number of oil bodies was noted. Changes in root cap (Fig. 5g, h) were the same as those observed in white lupine and were described above.
Ultrastructure of white lupine seedling cotyledons (a, b), hypocotyl (c, d), root meristem (e, f) and root cap (g, h). Seedlings were grown in vitro for 96 h on medium with 60 mM sucrose (+S; a, c, e, g) or without the sugar (−S; b, d, f, h). CW cell wall, M mitochondrion, Mu mucilage, OB oil body, S starch, SP storage protein, V vacuole
Ultrastructure of Andean lupine seedling cotyledons (a, b), hypocotyl (c, d), root meristem (e, f) and root cap (g, h). Seedlings were grown in vitro for 96 h on medium with 60 mM sucrose (+S; a, c, e, g) or without the sugar (−S; b, d, f, h). CW cell wall, M mitochondrion, Mu mucilage, OB oil body, P plastid, S starch, SP storage protein, V vacuole
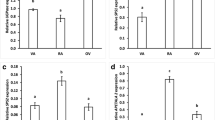
Soluble protein content in all studied organs of the three investigated lupine species decreased after 96 h of growth (comparing to organs of imbibed seeds; Fig. 6). In addition, significantly lower level of soluble protein was noted in each of organs grown on medium without sucrose (−S; Fig. 6).
Soluble protein content in yellow, white and Andean lupine embryo axes and cotyledons of dry and imbibed seeds (24 h) and in organs grown in vitro for 96 h on medium with 60 mM sucrose (+S) or without the sugar (−S). Small graphs are the magnification of soluble protein content in seeding axes and isolated embryo axes grown in vitro for 96 h. Statistical significance at p ≤ 0.05 (*) or at p ≤ 0.01 (**)
The activity of glutamate dehydrogenase in Andean lupine organs grown for 96 h was highest in the mitochondrial fraction. Its activity in the cytosolic fraction was very low and reach maximum of about 4% of the mitochondrial activity (Fig. 7). Glutamate dehydrogenase activity was significantly higher in each of the studied organs grown on −S medium, both in cytosolic and mitochondrial fractions (Fig. 7).
Discussion
The results of the experiments presented in this paper clearly showed that carbohydrates play a very important role as a regulator of metabolism of germinating seeds of yellow, white and Andean lupine. First of all, sucrose (60 mM) added to the medium of in vitro culture stimulated growth of isolated embryo axes and isolated cotyledons (Figs. 1b, c, 2b, c, 3b, c; Table 1), but growth of seedlings was significantly inhibited by sucrose (Figs. 1a, 2a, 3a; Table 1). Dry matter content was smaller in each of the studied organs grown on −S medium (Table 1). Inhibitory effect of sugars on germination and seedling growth has been already described in the literature. Exogenous sucrose (20 mM) causes slight delay in germination of Arabidopsis seeds (Rylott et al. 2001) but in higher concentration (330 mM) inhibits germination and seedling growth (Laby et al. 2000). Similar effect is caused by glucose which inhibits germination of seeds of Arabidopsis, delays seedling development and significantly hinders mobilization of the main storage compound, i.e. lipid (Gibson et al. 2001; To et al. 2002). Moreover, the effect of glucose is not due to osmotic stress (To et al. 2002).
Data presented in this paper proved that mobilization of storage compounds during lupine seeds germination was controlled by sugar level in tissues. Carbohydrate levels regulated the breakdown of not only main storage compound, i.e. protein, but also storage lipid and starch as well. Mature lupine seeds contain no starch, but it is accumulated in high amount during seeds imbibition and is mobilized during subsequent days of germination (Borek et al. 2006). Depletion in carbohydrates in tissues caused enhancement in storage compounds breakdown. Ultrastructure research showed that in lupine seedlings grown on medium without sucrose (−S), protein deposits were smaller, oil bodies were less numerous and starch granules were smaller or fewer. Those changes were very similar in seedlings of all three investigated lupine species, i.e. yellow (Borek et al. 2006), white (Fig. 4) and Andean lupine (Fig. 5). The content of soluble protein in each of the studied organs of three lupine species was lower when there was no sucrose in the medium (Fig. 6). During lupine seeds germination, respiration is decreased in sugar-deficient conditions (Morkunas et al. 2003; Borek et al. 2011a). Decreasing respiration in carbohydrate exhausting conditions has been observed also in other species, for example in glucose-starved excised root tips of maize (Brouquisse et al. 1991) or in sucrose-starved isolated embryo axes of pea (Morkunas et al. 2000). In such circumstances, it is very important to sustain respiration to ensure growth and development of seedlings. In such conditions, storage compounds and other cell components are faster degraded to provide respiratory substrates (Brouquisse et al. 1991; Yu 1999; Borek et al. 2001; Gonzali et al. 2006). One of the convenient respiratory substrates, especially in leguminous plants, is amino acids. It has been proved that amino acids (including glutamate) liberated from storage protein are used as respiratory substrates in yellow lupine (Ratajczak et al. 1996; Borek et al. 2001; Lehmann and Ratajczak 2008) and amino acids utilization is enhanced in sugar depletion conditions (Borek et al. 2001). Enhancement in glutamate utilization is also observed in sucrose-starved isolated embryo axes of pea (Morkunas et al. 2000). In sugar-depleted conditions in yellow lupine germinating seeds, an increased activity of exo- and endopeptidases has been noted (Borek and Ratajczak 2002). An increase in proteolytic activity during carbohydrate starvation has been also reported in the callus of Gerbera (Tassi et al. 1992) and in maize root tips (James et al. 1993). An increase in the activity of proteolytic enzymes is not solely related to the degradation of storage proteins but also with process of autophagy. The increased activity of proteolytic enzymes and the intensity of autolysis of cytoplasmic proteins in the absence of sugars may be an adaptation of cellular metabolism to ensure normal growth and development of plants. Such phenomenon can be observed in cell suspension of rice (Chen et al. 1994), cell suspension of tobacco (Moriyasu and Ohsumi 1996) and in cell suspension of sycamore (Journet et al. 1996). It is highly possible that sucrose starvation caused autophagy in lupine embryo axes as well. Such conclusion is based on increased vacuolization of cells of root meristematic zone (Fig. 4f). Increase in vacuolization indicates advanced autophagy (Marty 1999), which is a mechanism for degradation of cellular contents in order to recycle nutrients, especially protein (Thomson and Vierstra 2005). In plants, autophagy has been known to be important for nutrient remobilization during sugar and/or nitrogen starvation (Thomson and Vierstra 2005; Bassham 2007). Ultrastructure observations of root meristematic zone of lupine isolated embryo axes have showed considerable increase in vacuole number and size in sucrose-starved organs (Borek et al. 2006, 2011a). Increase in vacuolization was observed in root meristematic zone of seedling axes as well (Fig. 4f) where endogenous carbon source exists (cotyledons). The most evident increase in vacuolization was identified in the cells of white lupine axes, both in isolated embryo axes (Borek et al. 2011a) and in seedling axes (Fig. 4f). Other proof for autophagy in plant cells is a decrease in phosphatidylcholine content (Aubert et al. 1996; Inoue and Moriyasu 2006). Such decrease of this phospholipid has been observed in sucrose-starved lupine axes as well (Borek et al. 2011b). Issue concerning autophagy in lupine embryo axes has been discussed more detailed in our other papers (Borek et al. 2011a, b) and definitely needs further research. It is worth to add here that respiration is decreased in sugar-starved isolated embryo axes of yellow lupine (Morkunas et al. 2003; Borek et al. 2011a) but mitochondria are protected and not degraded (Morkunas et al. 2003).
The lupine seeds are rich in storage protein and large quantity of toxic ammonium is produced during its mobilization and amino acids metabolism. Nitrogen in this form is toxic (Britto and Kronzucker 2002) so its utilization is necessary. One of the possibilities of detoxification of ammonium is the synthesis of asparagine, content of which in germinating lupine seeds may reach 30% of the dry matter (Lehmann and Ratajczak 2008). Ammonium is assimilated to asparagine in two steps. At first, glutamine is synthesized in the reaction of the glutamate amination catalyzed by glutamine synthetase and than glutamine is transaminated with aspartate by asparagine synthetase. The product of this reaction is asparagine (Lehmann and Ratajczak 2008; Borek et al. 2011a). The main source of ammonium during lupine seeds germination is glutamate and its oxidative deamination catalyzed by glutamate dehydrogenase (Lehmann and Ratajczak 2008). Ammonium is produced in a catabolic action of glutamate dehydrogenase. The enzyme might function also anabolically in opposite direction into glutamate synthesis from 2-oxoglutarate and ammonium. The reversibility of the reaction catalyzed by glutamate dehydrogenase in vivo is considered as an alternative and depends on carbon and ammonium status (Lehmann et al. 2011). Activity of glutamate dehydrogenase in Andean lupine organs was assayed in the anabolic direction (synthesis of glutamate), because it is easier in vitro assays where the anabolic activity is about three times higher than the catabolic activity (Lehmann et al. 2011). Up to now, glutamate dehydrogenase has been intensively studied in germinating yellow lupine seeds (Ratajczak et al. 1996; Morkunas et al. 2000; Lehmann et al. 2003, 2010, 2011; Lehmann and Ratajczak 2008). During germination of yellow (Morkunas et al. 2000; Lehmann et al. 2003, 2010) and Andean (Fig. 7) lupine seeds, the activity of glutamate dehydrogenase was considerably higher in carbohydrate depletion conditions (−S). It is highly possible that increase in enzyme activity in both lupine species was caused by enhanced gene expression. There are many literature data proving that regulatory mechanism of sugar in plant metabolism is based on modifications in gene expression (Koch 1996; Gonzali et al. 2006; Rolland et al. 2006; Li et al. 2006; Ramon et al. 2008; Smeekens et al. 2010). In Arabidopsis seedlings, glucose (during 6 h of treatment) stimulates or inhibits the expression of about 6.6% of all genes (Li et al. 2006). Expression of the genes of glutamate dehydrogenase in Andean lupine was not investigated but in the literature it is strongly recommended that increase in catabolic activity of enzyme in carbohydrate depletion conditions is caused by enhanced gene expression. Such interpretation is based on changes in the isoenzymatic pattern of glutamate dehydrogenase in yellow lupine embryo axes and on the results of experiments using RNA and protein synthesis inhibitors (Morkunas et al. 2000; Lehmann et al. 2003, 2010).
Summarizing data presented in this paper, it is possible to conclude that carbohydrates play an important role in the regulation of metabolism of germinating yellow, white and Andean lupine seeds. Sucrose regulated growth and development of lupine seedling (as well as isolated organs) and control mobilization of storage compounds. Under conditions of carbohydrate depletion in tissues, enhancement in storage protein breakdown was observed. Regulation of storage protein mobilization by sucrose during lupine seeds germination was similar in all three investigated species and the results described herein are consistent with the results of research conducted on non-leguminous plants. However, induction of autophagy in carbohydrate-starved tissues of young embryo axes of germinating lupine seeds is a very interesting issue that needs further research.
References
Aubert S, Gout E, Bligny R, Marty-Mazars D, Barrieu F, Alabouvette J, Marty F, Douce R (1996) Ultrastructural and biochemical characterization of autophagy in higher plant cells subjected to carbon deprivation: control by the supply of mitochondria with respiratory substrates. J Cell Biol 133:1251–1263
Bassham DC (2007) Plant autophagy—more than a starvation response. Curr Opin Plant Biol 10:587–593
Bewley JD, Black M (1994) Seeds physiology of development and germination. Ed. II. Plenum Press, New York
Borek S, Nuc K (2011) Sucrose controls storage lipid breakdown on gene expression level in germinating yellow lupine (Lupinus luteus L.) seeds. J Plant Physiol 168:1795–1803
Borek S, Ratajczak W (2002) Sugars as a metabolic regulator of storage protein mobilization in germinating seeds of yellow lupine (Lupinus luteus L.). Acta Physiol Plant 24:425–434
Borek S, Ratajczak L (2010) Storage lipids as a source of carbon skeletons for asparagine synthesis in germinating seeds of yellow lupine (Lupinus luteus L.). J Plant Physiol 167:717–724
Borek S, Morkunas J, Ratajczak W, Ratajczak L (2001) Metabolism of amino acids in germinating yellow lupine seeds III. Breakdown of arginine in sugar-starved organs cultivated in vitro. Acta Physiol Plant 23:141–148
Borek S, Ratajczak W, Ratajczak L (2003) A transfer of carbon atoms from fatty acids to sugars and amino acids in yellow lupine (Lupinus luteus L.) seedlings. J Plant Physiol 160:539–545
Borek S, Ratajczak W, Ratajczak L (2006) Ultrastructural and enzymatic research on the role of sucrose in mobilization of storage lipids in germinating yellow lupine seeds. Plant Sci 170:441–452
Borek S, Pukacka S, Michalski K, Ratajczak L (2009) Lipid and protein accumulation in developing seeds of three lupine species: Lupinus luteus L., Lupinus albus L., and Lupinus mutabilis Sweet. J Exp Bot 60:3453–3466
Borek S, Kubala S, Kubala S, Ratajczak L (2011a) Comparative study of storage compound breakdown in germinating seeds of three lupine species. Acta Physiol Plant 33:1953–1968
Borek S, Pukacka S, Michalski K (2011b) Regulation by sucrose of storage compounds breakdown in germinating seeds of yellow lupine (Lupinus luteus L.), white lupine (Lupinus albus L.) and Andean lupine (Lupinus mutabilis Sweet). II. Mobilization of storage lipid. Acta Physiol Plant (in review)
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Britto DT, Kronzucker HJ (2002) NH4 + toxicity in higher plants: a critical review. J Plant Physiol 159:567–584
Brouquisse R, James F, Raymond P, Pradet A (1991) Study of glucose starvation in excised maize root tips. Plant Physiol 96:619–626
Cerletti P (1982) Lupin seeds proteins. In: Hudson BIF (ed) Development in food proteins. Applied Science Publisher LTD, pp 133–171
Chen MH, Liu LF, Chen YR, Wu HK, Yu SM (1994) Expression of α-amylases, carbohydrate metabolism, and autophagy in cultured rice cells is coordinately regulated by sugar nutrient. Plant J 6:625–636
Duranti M, Consonni A, Magni C, Sessa F, Scarafoni A (2008) The major proteins of lupin seed: characterisation and molecular properties for use as functional and nutraceutical ingredients. Trends Food Sci Tech 19:624–633
Gibson SI, Laby RJ, Kim D (2001) The sugar-insensitive1 (sis1) mutant of Arabidopsis is allelic to ctr1. Biochem Biophys Res Commun 280:196–203
Gonzali S, Loreti E, Solfanelli C, Novi G, Alpi A, Perata P (2006) Identification of sugar-modulated genes and evidence for in vivo sugar sensing in Arabidopsis. J Plant Res 119:115–123
Gwóźdź EA (1988) Biosynthesis of storage proteins in developing lupin seeds. In: Proceedings of 5th international Lupin Conference, pp 342–357
Hedley CL (2001) Grain legume carbohydrates. In: Hedley CL (ed) Carbohydrates in grain legume seeds: improving nutritional quality and agronomic characteristics. CAB International, Wallingford, pp 11–14
Heller R (1954) Recherches sur la nutrition minérale des tissus végétaux ciltivés in vitro. Ann Sci Nat Bot Biol Veg 14:1–223
Inoue Y, Moriyasu Y (2006) Autophagy is not a main contributor to the degradation of phospholipids in tobacco cells cultured under sucrose starvation conditions. Plant Cell Physiol 47:471–480
James F, Brouquisse R, Pradet A, Raymond P (1993) Changes in proteolytic activities in glucose-starved maize root tips: regulation by sugars. Plant Physiol Biochem 31:845–856
Journet EP, Bligny R, Douce R (1996) Biochemical changes during sucrose deprivation in higher plant cells. J Biol Chem 261:3193–3198
Karnovsky MJ (1965) A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Biol 27:137–138
Koch KE (1996) Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol 47:509–540
Laby RJ, Kincaid MS, Kim D, Gibson SI (2000) The Arabidopsis sugar-insensitive mutants sis4 and sis5 are defective in abscisic acid synthesis and response. Plant J 23:587–596
Lehmann T, Ratajczak L (2008) The pivotal role of glutamate dehydrogenase (GDH) in the mobilization of N and C from storage material to asparagine in germinating seeds of yellow lupine. J Plant Physiol 165:149–158
Lehmann T, Ratajczak L, Deckert J, Przybylska M (2003) The modifying effect of sucrose on glutamate dehydrogenase (GDH) activity in lupine embryos treated with inhibitors of RNA and protein synthesis. Acta Physiol Plant 25:325–335
Lehmann T, Skrok A, Dabert M (2010) Stress-induced changes in glutamate dehydrogenase activity imply its role in adaptation to C and N metabolism in lupine embryos. Physiol Plant 138:35–47
Lehmann T, Dabert M, Nowak W (2011) Organ-specific expression of glutamate dehydrogenase (GDH) subunits in yellow lupine. J Plant Physiol 168:1060–1066
Li Y, Lee KK, Walsh S, Smith C, Hadingham S, Sorefam K, Cawlej G, Bevan MW (2006) Establishing glucose- and ABA-regulated transcription networks in Arabidopsis by microarray analysis and promoter classification using a Relevance Vector Machine. Genome Res 16:414–427
Marty F (1999) Plant vacuoles. Plant Cell 11:587–599
Mohamed AA, Rayas-Duarte P (1995) Composition of Lupinus albus. Cereal Chem 72:643–647
Moriyasu Y, Ohsumi Y (1996) Autophagy in tobacco suspension cultured cells in response to sucrose starvation. Plant Physiol 111:1233–1241
Morkunas I, Lehmann T, Ratajczak W, Ratajczak L, Tomaszewska B (2000) The involvement of glutamate dehydrogenase in the adaptation of mitochondria to oxidize glutamate in sucrose starved pea embryos. Acta Physiol Plant 22:389–394
Morkunas I, Garnczarska M, Bednarski W, Ratajczak W, Waplak S (2003) Metabolic and ultrastructural responses of lupine embryo axes to sugar starvation. J Plant Physiol 160:311–319
Ramon M, Rolland F, Sheen J (2008) Sugar sensing and signaling. The Arabidopsis Book, American Society of Plant Biologists, doi:10.1199/tab.0117
Ratajczak W, Lehmann T, Polcyn W, Ratajczak L (1996) Metabolism of amino acids in germinating yellow lupin seeds. I. The decomposition of 14C-aspartate and 14C-glutamate during the imbibition. Acta Physiol Plant 18:13–18
Ratajczak W, Borek S, Podgórski A, Ratajczak L (1999) Variability of globulin composition in cultivars and individually tested seeds of yellow lupin (Lupinus luteus L.). Acta Physiol Plant 21:413–417
Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57:675–709
Rylott EL, Hooks MA, Graham IA (2001) Co-ordinate regulation of genes involved in storage lipid mobilization in Arabidopsis thaliana. Biochem Soc Trans 29:283–287
Santos CN, Ferreira RB, Teixeira AR (1997) Seeds proteins of Lupinus mutabilis. J Agric Food Chem 45:3821–3825
Smeekens S, Jingkun M, Johannes H, Rolland F (2010) Sugar signals and molecular networks controlling plant growth. Curr Opin Plant Biol 13:274–279
Spurr AR (1969) A low viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43
Tassi F, Maestri E, Restivo FM, Marminoli N (1992) The effects of carbon starvation on cellular metabolism and protein and RNA synthesis in Gerbera callus cultures. Plant Sci 83:127–136
Thomson AR, Vierstra RD (2005) Autophagic recycling: lessons from yeast help define the process in plants. Curr Opin Plant Biol 8:165–173
To JPC, Reiter WD, Gibson SI (2002) Mobilization of seed storage lipid by Arabidopsis seedlings is retarded in the presence of exogenous sugars. BMC Plant Biol 2:4
Watanabe M, Toshihiko H, Kikuchi A, Watanabe Y (1999) Purification and characterization of two glutamate dehydrogenase isoenzymes from Brassica napus. Plant Physiol Biochem 37:731–739
Yu SM (1999) Cellular and genetic responses of plants to sugar starvation. Plant Physiol 121:687–693
Acknowledgments
We thank Dr. Stanisław Stawiński, Head of Plant Breeding Station Smolice Division in Przebędowo (Murowana Goślina, Poland), for providing seeds of lupine. This work was partially supported by grant no. 2 P06A 004 29 from Polish science funding in years 2005–2008.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Weidner.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Borek, S., Kubala, S. & Kubala, S. Regulation by sucrose of storage compounds breakdown in germinating seeds of yellow lupine (Lupinus luteus L.), white lupine (Lupinus albus L.) and Andean lupine (Lupinus mutabilis Sweet): I. Mobilization of storage protein. Acta Physiol Plant 34, 701–711 (2012). https://doi.org/10.1007/s11738-011-0870-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-011-0870-1