Abstract
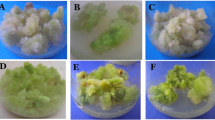
Genetic stability and phytochemical analysis of in vitro established plants of Picrorhiza kurroa Royle ex Benth, have been carried out. Random amplified polymorphic DNA (RAPD) and inter simple sequence repeats (ISSR) markers were used to assess the genetic fidelity of tissue culture products including three adventitious shoots from three calli and 6 months old tissue culture raised plants growing in green house condition with mother plant. Apparent genetic variation was detected in the five types of plant materials. The percentage of polymorphic bands in the RAPD and ISSR analysis were 16.25 and 14.54 %, respectively. The genetic similarity was calculated on the basis of RAPD and ISSR data among the five types of plant materials and were ranged from 0.5 to 1.0 (mean 0.75) and 0.47 to 1.0 (mean 0.73), respectively. The similarity coefficient by both RAPD and ISSR analysis revealed that differences between tissue culture raised plants and mother plant was not remarkable, but notable differences were observed among three adventitious shoots regenerated from three calli. The phytochemical analysis of tissue culture raised products showed higher secondary metabolite (picrotin and picrotoxinin) content as compare to mother plant. The information gained on genetic stability/variability will be valuable for the large scale propagation and secondary metabolite production of P. kurroa.



Similar content being viewed by others
Abbreviations
- MS:
-
Murashige and Skoog’s medium
- BAP:
-
6-Benzylaminopurine
- IAA:
-
Indole acetic acid
- NAA:
-
α-Naphthaleneacetic acid
- PCR:
-
Polymerase chain reaction
- RAPD:
-
Random amplified polymorphic DNA
- ISSR:
-
Inter simple sequence repeats
References
Agnihotri RK, Mishra J, Nandi SK (2009) Improving in vitro shoot multiplication and rooting and subsequent field evaluation of genetically uniform regenerants of Dendrocalamus hamiltonii. Acta Physiol Plant 31:961–967
Amoo SO, Aremu AO, Staden JV (2012) In vitro plant regeneration, secondary metabolite production and antioxidant activity of micropropagated Aloe arborescens Mill. Plant Cell Tiss Organ Cult. doi:10.1007/s11240-012-0200-3
Anand S (2010) Various approaches for secondary metabolite production through plant tissue culture. Pharmacia 1(1):1–7
Chandra B, Palni LMS, Nandi SK (2006) Propagation and conservation of Picrorhiza kurroa Royle ex Benth.: An endangered Himalayan medicinal herb of high commercial value. Biodivers Conserv 15:2325–2338
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Fujita Y (1988) Shikonin production by plant (Lithospermum erythrorhizon) cell cultures. In: Bajaj YPS (ed) Biotechnology in agriculture and forestry: medicinal and aromatic plants I, vol 4. Springer, Berlin
Fujita Y, Tabata M (1987) Secondary metabolites from plant cells: pharmaceutical applications and progress in commercial production. In: Somers DA, Hackett WP, Biesboer DD, Green CE (eds) Plant tissue and cell culture. Alan R Liss, New York
Giri L, Jugran A, Rawat S, Dhyani P, Andola H, Bhatt ID, Rawal RS, Dhar U (2012) In vitro propagation, genetic and phytochemical assessment of Habenaria edgeworthii: an important Astavarga plant. Acta Physiol Plant. doi:10.1007/s11738-011-0884-8
Guo B, Gao M, Liu CZ (2007) In vitro propagation of an endangered medicinal plant Saussurea involucrate Kar. et Kir. Plant Cell Rep 26:261–265
Hussain A (1984) Conservation of genetic resources of medicinal plants in India. In: Jain SK (ed) Conservation of tropical plant resources. Botanical Survey of India, Howrah, pp 110–117
Isabel NL, Tremblay MM, Tremblay FM, Bousquet J (1993) RAPD as an aid to evaluate the genetic integrity of somatic embryogenesis derived population of Picea mariana (Mill). Theor Appl Genet 86:81–87
Kawiak A, Lojkowaska E (2004) Application of RAPD in the determination o genetic fidelity in micropropagated Drosera plantlets. In Vitro Cell Dev Biol Plant 40:592–595
Kirtikar KR, Basu BD (1984) Indian medicinal plants, vol 3. Bishen Singh Mahendra Pal Singh, Dehradun, pp 1824–1826
Lal N, Ahuja PS, Kukreja AK, Pandey B (1988) Clonal propagation of Picrorhiza kurroa Royle ex Benth. by shoot tip culture. Plant Cell Rep 7:201–205
Larkin P, Scowcroft W (1981) Somaclonal variation—a novel source of variability from cell culture for plant improvement. Theor Appl Genet 60:197–214
Mehrotra S, Khwaja O, Kukreja AK, Rahman L (2012) ISSR and RAPD based evaluation of genetic stability of encapsulated microshoots of Glycyrrhiza glabra following 6 months of storage. Mol Biotechnol. doi:10.1007/s12033-011-9491-6
Mishra J, Bhandari H, Singh M, Rawat S, Agnihotri RK, Mishra S, Purohit S (2011a) Hairy root culture of Picrorhiza kurroa Royle ex Benth.: a promising approach for the production of picrotin and picrotoxinin. Acta Physiol. Plant 33(5):1841–1846. doi:10.1007/s11738-011-0724-x
Mishra J, Singh M, Palni LMS, Nandi SK (2011b) Assessment of genetic fidelity of encapsulated microshoots of Picrorhiza kurroa. Plant Cell Tiss Org Cult 104:181–186
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15:473–497
Nei M, Li WH (1979) Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Nat Acad Sci USA 76:5269–5273
Petiard V, Baubault C, Bariaud A, Hutin M, Courtois D (1985) Studies on variability of plant tissue cultures for alkaloid production in Catharanthus roseus and Papaver somniferum callus cultures. In: Neumann N (ed) Primary and Secondary Metabolism of Plant Cell Cultures. Springer, Berlin
Rahman MH, Rajora OP (2001) Microsatellite DNA somaclonal variation in micropropagated trembling aspen (Populus tremuloides). Plant Cell Rep 20:531–536
Rani V, Raina SN (2000) Genetic fidelity of organized meristem-derived micropropagated plants: a critical reappraisal. In Vitro Cell Dev Biol Plant 36:319–330
Rani V, Ajay P, Raina SN (1995) Random amplified polymorphic DNA (RAPD) markers for genetic analysis in micropropagated plants of Populus deltoides Marsh. Plant Cell Rep 14:459–462
Rawat B, Rawat-Mishra J, Mishra S, Mishra SN (2013) Picrorhiza kurroa: current status and tissue culture based biotechnological interventions. Acta Physiol Plant. doi:10.1007/s11738-012-1069-9
Samant SS, Dhar U, Palni LMS (1998) Medicinal plants of Indian Himalaya: diversity, distribution and potential value. Gyanodaya Prakashan, Nainital (p 163)
Scowcroft W (1985) Somaclonal variation: The myth of clonal uniformity. In: Hohn B, Dennis ES (eds) Genetic flux in plants. Springer, Vienna
Skirvin RM, McPheeters KD, Norton M (1994) Sources of frequency of somaclonal variation. Hort Sci 29:1232–1237
Thakur RS, Puri HS, Hussain A (1989) Major medicinal plants of India. CIMAP, Lucknow, pp 404–407
Ushiyama K (1991) Large scale culture of ginseng. In: Komamine A, Misawa M, DiCosmo F (eds) Plant cell culture in Japan. CMC, Tokyo
Ved DK, Kinhal GA, Ravikumar K, Prabhakaran V, Ghate U, Sankar RV, Indresha JH (2003) Conservation assessment and management prioritization for the medicinal plants of Himachal Pradesh, Jammu and Kashmir and Uttaranchal. Foundation for Revitalization of Local Health Traditons, Bangalore
Williams K, Kubelik AR, Rafalski JA, Tingey SV (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acid Res 18:1631–1635
Yesil-Celiktas O, Girgin G, Orhan H, Wichers HJ, Bedir E, Vardar- Sukan F (2007) Screening of free radical scavenging capacity and antioxidant activities of Rosmarinus officinalis extracts with focus on location and harvesting times. Eur Food Res Technol 224:443–451
Zietkiewicz E, Rafalski A, Labuda D (1994) Genome fingerprinting by simple sequence repeats (SSR)—anchored polymerase chain reaction amplification. Genomics 20:176–183
Acknowledgments
Director of the Institute is thanked for providing facilities. The financial assistance to the author JMR from Department of Science and Technology (WOS/LS-191/2010), India is gratefully acknowledged. Sincere thanks goes to CSIR (09/560(0015)/2011-EMR-I) for financial and technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. W. Sadowski.
Rights and permissions
About this article
Cite this article
Rawat, J.M., Rawat, B., Mehrotra, S. et al. ISSR and RAPD based evaluation of genetic fidelity and active ingredient analysis of regenerated plants of Picrorhiza kurroa . Acta Physiol Plant 35, 1797–1805 (2013). https://doi.org/10.1007/s11738-013-1217-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-013-1217-x