Abstract
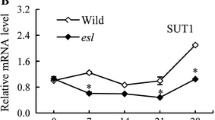
The upper leaf sheath of rice (Oryza sativa L.) serves as a temporary starch sink before heading, subsequently becoming a carbon source tissue to the growing panicle at the post-heading stage. The time of sink–source transition in upper leaf sheaths is highly correlated to the panicle exsertion. Here, we found that the expression profiles of starch synthesis genes such as ADP-glucose pyrophosphorylase large subunit 2, granule-bound starch synthase II, soluble starch synthase I, starch branching enzyme (SBE) I, SBEIII, and SBEIV were highly correlated with starch content changes during the heading period in the second leaf sheath below the flag leaf. In addition, the α-amylase2A and β-amylase were considered as major genes that were in charge of starch degradation at the post-heading period. Of the five sucrose transporter (OsSUT) genes, OsSUT1 and OsSUT4 appeared to play an important role in sucrose loading into the phloem of source leaf sheaths. Moreover, the microarray-based data implied that the dominant processes associated with functional leaf sheath transition from sink to source were carbohydrate metabolism and the translocation of the carbon and nitrogen sources and inorganic phosphate.







Similar content being viewed by others
Abbreviations
- AGPase:
-
ADP-glucose pyrophosphorylase
- DBE:
-
Debranching enzyme
- GBSS:
-
Granule-bound starch synthase
- SBE:
-
Starch branching enzyme
- SSS:
-
Soluble starch synthase
- SUT:
-
Sucrose transporter
References
Akihiro T, Mizuno K, Fujimura T (2005) Gene expression of ADP-glucose pyrophosphorylase and starch contents in rice cultured cells are cooperatively regulated by sucrose and ABA. Plant Cell Physiol 46:937–946
Aoki N, Scofield GN, Wang XD, Patrick JW, Offler CE, Furbank RT (2003) The sucrose transporter gene family in rice. Plant Cell Physiol 44:223–232
Blum A, Sinmena B, Mayer J, Golan G, Shpiler L (1994) Stem reserve mobilization supports wheat-grain filling under heat stress. Aust J Plant Physiol 21:771–781
Chu C, Lee TM (1989) The relationship between ethylene biosynthesis and chilling tolerance in seedlings of rice (Oryza sativa L.). Bot Bull Acad Sinica 30:263–273
Cock JH, Yoshida S (1972) Accumulation of 14C-labelled carbohydrate before flowing and its subsequent redistribution and respiration in the rice plant. Proc Crop Sci Soc Jpn 41:226–234
Déjardin A, Sokolov LN, Kleczkowski LA (1999) Sugar/osmoticum levels modulate differential abscisic acid-independent expression of two stress-responsive sucrose synthase genes in Arabidopsis. Biochem J 344:503–509
Dian W, Jiang H, Wu P (2005) Evolution and expression analysis of starch synthase III and IV in rice. J Exp Bot 56:623–632
Dingkuhn M, Schnier HF, De Datta SK, Dorffling K, Javellana C (1991) Relationship between ripening-phase productivity and crop duration, canopy photosynthesis and senescence in transplanted and direct-seeded lowland rice. Field Crops Res 26:327–345
Gao Z, Maurousset L, Lemoine R, Yoo SD, Nocker S, Loescher W (2003) Cloning, expression, and characterization of sorbitol transporters from developing sour cherry fruit and leaf sink tissues. Plant Physiol 131:1–10
Hirose T, Terao T (2004) A comprehensive expression analysis of the starch synthase gene family in rice (Oryza sativa L.). Planta 220:9–16
Hirose T, Endler A, Ohsugi R (1999) Gene expression of enzymes for starch and sucrose metabolism and transport in leaf sheaths of rice (Oryza sativa L.) during the heading period in relation to the sink to source transition. Plant Prod Sci 2:178–183
Hirose T, Ohdan T, Nakamura Y, Terao T (2006) Expression profiling of genes related to starch synthesis in rice leaf sheaths during the heading period. Physiol Plant 128:425–435
Ishikawa T, Akita S, Li Q (1993) Relationship between contents of non-structural carbohydrates before panicle initiation stage and grain yield in rice (Oryza sativa L.). Jpn J Crop Sci 62:130–131
Ishimaru K, Hirose T, Aoki N, Takahashi S, Ono K, Yamamoto S, Wu J, Saji S, Baba T, Ugaki M, Matsumotoi T, Ohsugi R (2001) Antisense expression of a rice sucrose transporter OsSUT1 in rice (Oryza sativa L.). Plant Cell Physiol 42:1181–1185
Ishimaru K, Kosone M, Sasaki H, Kashiwagi T. (2004) Leaf contents differ depending on the position in a rice leaf sheath during sink–source transition. Plant Physiol Biochem 42:855–860
Ishizuka Y, Tanaka A (1953) Biochemical studies on the life history of rice plants I. Absorption and translocation of inorganic elements. J Sci Soil Manure Jpn 23:23–28
Karrer EE, Rodriguez RL (1992) Metabolic regulation of rice a-amylase and sucrose synthase gene in planta. Plant J 2:517–523
Keppler D, Decker K (1974) Glycogen: determination with amyloglucosidase. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol 3. Academic Press, New York, pp 1127–1131
Kumar A, Silim SN, Okamoto M, Siddiqi MY, Glass AD (2003) Differential expression of three members of the AMT1 gene family encoding putative high-affinity NH4 + transporters in roots of Oryza sativa subspecies indica. Plant Cell Environ 26:907–914
Lazan H, Ng SY, Goh LY, Ali ZM (2004) Papaya beta-galactosidase/galactanase isoforms in differential cell wall hydrolysis and fruit softening during ripening. Plant Physiol Biochem 42:847–853
Mae T, Ohira K (1981) The remobilization of nitrogen related to leaf growth and senescence in rice plants (Oryza sativa L.) Plant Cell Physiol 22:1067–1074
Mizuno K, Kawasaki T, Shimada H, Satoh H, Kobayashi E, Okumura S, Arai Y, Baba T (1993) Alteration of the structural properties of starch components by the lack of an isoform of starch branching enzyme in rice seeds. J Biol Chem 268:19084–19091
Muñoz FJ, Baroja-Fernandez E, Moran-Zorzano MT, Viale AM, Etxeberria E, Alonso-Casajus N, Pozueta-Romero J (2005) Sucrose synthase controls both intracellular ADP glucose levels and transitory starch biosynthesis in source leaves. Plant Cell Physiol 46:1366–1376
Nakano H, Makino A, Mae T (1995) Effects of panicle removal on the photosynthetic characteristics of the flag leaf of rice plants during the ripening stage. Plant Cell Physiol 36:653–659
Nakamura Y, Takeichi T, Kawaguchi K, Yamanouchi H (1992) Purification of two forms of starch branching enzyme (Q-enzyme) from developing rice endosperm. Physiol Plant 84:329–335
Ohdan T, Francisco PB, Sawada T, Hirose T, Terao T, Satoh H, Nakamura Y (2005) Expression profiling of genes involved in starch synthesis in sink and source organs of rice. J Exp Bot 56:3229–3244
Perez CM, Palmiano EP, Baun LC, Juliano BO (1971) Starch metabolism in the leaf sheaths and culm of rice. Plant Physiol 47:404–408
Samonte SOPB, Wilson LT, McClung AM, Tarpley L (2001) Seasonal dynamics of nonstructural carbohydrate partitioning in fifteen diverse rice (Oryza sativa L.) genotypes. Crop Sci 41:902–909
Scofield GN, Hirose T, Gaudron JA, Upadhyaya NM, Ohsugi R, Furbank RT (2002) Antisense suppression of the rice sucrose transporter gene, OsSUT1, leads to impaired grain filling and germination but does not affect photosynthesis. Funct Plant Biol 29:815–826
Smith FW, Mudge SR, Rae AL, Glassop D (2003) Phosphate transport in plants. Plant Soil 248:71–83
Smith AM, Zeeman SC, Smith SM (2005) Starch degradation. Annu Rev Plant Biol 56:73–98
Sokolov LN, Déjardin A, Kleczkowski LA (1998) Sugars and light/dark exposure trigger differential regulation of ADP-glucose pyrophosphorylase genes in Arabidopsis thaliana (thale cress) Biochem J 336:681–687
Sonoda Y, Ikeda A, Saiki S, Yamaya T, Yamaguchi J (2003) Feedback regulation of the ammonium transporter gene family AMT1 by glutamine in rice. Plant Cell Physiol 44:1396–1402
Suenaga A, Moriya K, Sonoda Y, Ikeda A, Von Wiren N, Hayakawa T, Yamaguchi J, Yamaya T (2003) Constitutive expression of a novel-type ammonium transporter OsAMT2 in rice plants. Plant Cell Physiol 44:206–211
Takahashi S, Ishimaru K, Yazaki J, Fujii F, Shimbo K, Yamamoto K, Sakata K, Sasaki T, Kishimoto N, Kikuchi S (2005) Microarray analysis of sink–source transition in rice leaf sheaths. Breed Sci 55:153–162
Tanaka A (1961) Studies on the nutrio-physiology of leaves of rice. Plant. J Fac Agric Hokkaido Univ 51:449–550
Tetlow IJ, Morell MK, Emes MJ (2004) Recent developments in understanding the regulation of starch metabolism in higher plants. J Exp Bot 55:2131–2145
Thomas BR, Rodriguez RL (1994) Metabolite signals regulate gene expression and source/sink relations in cereal seedlings. Plant Physiol 106:1235–1239
Varghese JN, Hrmova M, Fincher GB (1999) Three-dimensional structure of a barley β-d-glucan exohydrolase, a family 3 glycosyl hydrolase. Structure 7:179–190
Wang MY, Siddiqi MY, Ruth TJ, Glass ADM (1993) Ammonium uptake by rice roots. II. Kinetics of 13NH4 + influx across the plasmalemma. Plant Physiol 103:1259–1267
Watanabe Y, Nakamura Y, Ishii R (1997) Relationship between starch accumulation and activities of the related enzymes in the leaf sheath as a temporary sink organ in rice (Oryza sativa). Aust J Plant Physiol 24:563–569
Wright KM, Roberts AG, Martens HJ, Sauer N, Oparka KJ (2003) Structural and functional vein maturation in developing tobacco leaves in relation to AtSUC2 promoter activity. Plant Physiol 131:1555–1565
Yang J, Zhang J, Wang Z, Xu G, Zhu Q (2004) Activities of key enzymes in sucrose-to-starch conversion in wheat grains subjected to water deficit during grain filling. Plant Physiol 135:1621–1629
Yoshida S (1972) Physiological aspects of grain yield. Annu Rev Plant Physiol 3:437–464
Acknowledgments
We thank Dr. L.F. Liu and Dr. H. S. Lur (National Taiwan University, Taipei, Taiwan) for their helpful comments and discussions. This research was supported by the National Science Council, Taiwan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Janska.
Rights and permissions
About this article
Cite this article
Chen, HJ., Wang, SJ. Molecular regulation of sink–source transition in rice leaf sheaths during the heading period. Acta Physiol Plant 30, 639–649 (2008). https://doi.org/10.1007/s11738-008-0160-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-008-0160-8