Abstract
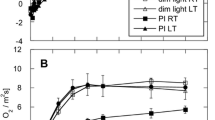
The respiration rate of leaves and mesophyll protoplasts of pea (Pisum sativum L.), from plants which were previously kept in darkness for 24 h was doubled following a period of photosynthesis at ambient level of O2 (21 %), whereas the low level of O2 (1 % and 4 % for leaves and protoplasts, respectively) reduced this light-enhanced dark respiration (LEDR) to the rate as noted before the illumination. Similarly to respiration rate, the oxygen at used concentrations had no effect on the ATP/ADP ratio in the dark-treated leaves. However, the ATP/ADP ratio in leaves photosynthesizing at 21 % O2 was higher (up to 40 %, dependence on CO2 concentration in the range 40–1600 1 dm−3) than in those photosynthesizing at 1 % O2 or darkened at air (21 % O2). Also, at 1 % O2 the accumulation of malate was suppressed (by about 40 %), to a value noted for leaves darkened at 21 % O2. The dark-treatment of leaves reduced the ability of isolated mitochondria to oxidize glycine (by about twofold) and succinate, but not malate. Mitochondria from both the light- and dark-treated leaves did not differ in qualitative composition of free amino acids, however, there were significant quantitative differences especially with respect to aspartate, alanine, glutamate and major intermediates of the photorespiratory pathway (glycine, serine). Our results suggest that accumulation of photorespiratory and respiratory metabolites in pea leaves during photosynthesis at 1 % O2 is reduced, hence the suppression of postillumination respiration rate.
Similar content being viewed by others
Abbreviations
- BSA:
-
bovine serinum albumin
- EDTA:
-
ethylenediaminetetra acetic acid
- GDC:
-
glycine decarboxylase
- HEPES:
-
N-(2-hydroxyethyl)piperazine-N′-2(-ethanesulfonic acid)
- LEDR:
-
light-enhanced dark respiration
- MDH:
-
malate dehydrogenase
- ME:
-
malic enzyme
- MOPS:
-
3-(N-morpholino)propane sulfonic acid)
- PDC:
-
pyruvate dehydrogenase
- PFD:
-
photosynthetic photon flux density
- PVP:
-
polyvinylpyrrolidone
- RCR:
-
respiratory control rate
- TCA:
-
tricarboxylic acid cycle
- Tris:
-
tri(hydroxymethyl)methyloamine
References
Atkin O.K., Cummins W.R., Collier D.E. 1993. Light induction of alternative pathway capacity in leaf slices of Belgium endive. Plant Cell Environ., 16: 231–235.
Atkin O.K., Evans J.R., Siebke K. 1998. Relationship between the inhibition of leaf respiration and enhancement of leaf dark respiration following light treatment. Aust. J. Plant. Physiol., 25: 437–443.
Azcón-Bieto J., Lambers H., Day D.A. 1983. Effect of photosynthesis and carbohydrate status on respiratory rates and the involvement of the alternative pathway in leaf respiration. Plant Physiol., 72: 598–603.
Azcón-Bieto J., Osmond C.B. 1983. Relationship between photosynthesis and respiration. The effect of carbohydrate status on the rate of CO2 production by respiration in darkened and illuminated wheat leaves. Plant Physiol., 71: 574–578.
Bradford M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 72: 248–254.
Bruhn D., Mikkelsen T.N., Atkin O.K. 2002. Does the direct effect of atmospheric CO2 concentration on leaf respiration vary with temperature? Responses in two species of Plantago that differ in relative growth rate. Physiol. Plant., 114: 57–64.
Coudret A., Ferron F., Gaudillre J.P. 1981. Photosynthates formation in wheat under different partial oxygen pressures and temperatures. Photosynthetica 15: 21–27.
Day D.A., Whelan J., Millar A.H., Siedow J.N., Wiskich J.T. 1995. Regulation of the alternative oxidase in plant and fungi. Aust. J. Plant Physiol., 22: 497–509.
Farineau J. 1999. Study of the non-photochemical dark rise in chlorophyll fluorescence in pre-illuminated leaves of various C3 and C4 plants submitted to partial anaerobiosis. Plant Physiol. Biochem., 37: 911–918.
Feild T.S., Nedbal L., Ort D.R. 1998. Nonphotochemical reduction of the plastoquinone pool in sunflower leaves originates from chlororespiration. Plant Physiol., 116: 1209–1218.
Gale J., Reuveni J., Mayer., A.M. 1992. An oxygen gradient method for determining the partitioning of respiration between its cytochrome and alternative pathway components, with application to Lemna gibba fronds under different temperatures. In: Molecular, Biochemical and Physiological Aspects of Plant Respiration, ed. by H. Lambers, L.H.W. van der Plas, SPB Academic Publ., The Hague: 433–442.
Gardeström P., Wigge B. 1988. Influence of photorespiration on ATP/ADP ratios in the chloroplasts, mitochondria and cytosol, studied by rapid fractionation of barley (Hordeum vulgare) protoplasts. Plant Physiol., 88: 69–76.
Gonzlez-Meler M.A., Ribas-Carbó M., Siedow J.N., Drake B.G. 1996. Direct inhibition of plant mitochondrial respiration by elevated CO2. Plant Physiol., 112: 1349–1355.
Heldt H.W., Raghavendra A.S., Reumann S., Bettermann M., Hanning I., Benz R., Maier E. 1998. Redox transfer between mitochondria and peroxisomes in photorespiration. In: Plant Mitochondria: From Gene to Function, Ed. by I.M. Miller, P. Gardeström, K. Glimelius, E. Glaser, Backhuys Publ., Leiden: 543–549.
Hill S.A., Bryce J.H. 1992. Malate metabolism and light-enhanced dark respiration in barley mesophyll protoplasts. In: Molecular, Biochemical and Physiological Aspects of Plant Respiration, ed. by H. Lambers, L.H.W. van der Plas. SPB Academic Publ., The Hague: 221–230.
Hohorst H.J. 1965. L-(-)-Malate. Determination with malic dehydrogenase and DPN. In: Methods of enzymatic analysis, ed. by H.U. Bergmeyer, Verlag Chemie GMBH: 328–332.
Igamberdiev A.U., Bykova N.V., Gardeström P. 1997. Involvement of cyanide-resistant and rotenone-insensitive pathways of mitochondrial electron transport during oxidation of glycine in higher plants. FEBS Lett., 412: 265–269.
Igamberdiev A.U., Romanowska E., Gardeström P. 2001. Photorespiratory flux and mitochondrial contribution to energy and redox balance of barley leaf protoplasts in the light and during light-dark transitions. J. Plant Physiol., 158: 1325–1332.
Joët T., Cournac L., Peltier G., Havaux M. 2002. Cyclic electron flow around photosystem I in C3 plants. In vivo control by the redox state of chloroplasts and involvement of the NADH-dehydrogenase complex. Plant Physiol., 128: 760–769.
Lichtenthaler H.K., Wellburn R.R. 1983. Determination of total carotenoids and chlorophyll a and b on leaf extracts in different solvents. Biochem. Soc. Trans., 603: 591–592.
McClung C.R., Hsu M., Painter J.E., Gagne J.M., Karlsberg S.D., Salomé P.A. 2000. Integrated temporal regulation of the photorespiratory pathways. Circadian regulation of two Arabidopsis genes encoding serine hydroxymethyltransferase. Plant Physiol., 123: 381–391.
Miyachi S. 1979. Light-enhanced dark CO2 fixation. In: Encyclopedia of Plant Physiology, Photosynthesis II, Photosynthetic carbon metabolism and related processes, ed by M. Gibbs, E. Latzko, vol. 6, Springer-Verlag, Berlin, Heidelberg: 68–76.
Oestreicher G., Hogue P., Singer T.P. 1973. Regulation of succinate dehydrogenase in higher plants. II. Activation by substrates, reduced coenzyme Q, nucleotides, and anions. Plant Physiol., 52: 622–626.
Padmasree K., Raghavendra A.S. 1999a. Importance of oxidative electron transport over oxidative phosphorylation in optimizing photosynthesis in mesophyll protoplasts of pea (Pisum sativum L.). Physiol. Plant., 105: 546–553.
Padmasree K., Raghavendra A.S. 1999b. Response of photosynthetic carbon assimilation in mesophyll protoplasts to restriction on mitochondrial oxidative metabolism: Metabolites related to the redox status and sucrose biosynthesis. Photosynth. Res., 62: 231–239.
Parys E., Romanowska E. 2000. Relationship between postillumination burst of CO2 and enhancement of respiration in tall fescue leaves. Acta Physiol. Plant., 22: 135–142.
Parys E., Romanowska E., Lechowicz W., Poskuta J. 1989. Postillumination burst of CO2 in maize and reed grass leaves determined in a closed gas exchange system. Plant Physiol. Biochem., 27: 27–34.
Parys E., Romanowska E., Siedlecka M., Poskuta J.W.P. 1998. The effect of lead on photosynyhesis and respiration in detached leaves and in mesophyll protoplasts of Pisum sativum. Acta Physiol. Plant., 20: 313–322.
Parys E., Romanowska E., Siedlecka M. 2001. The effect of illumination and oxygen concentration on respiration and metabolites content in pea leaves. In: PS2001 Proc. 12th International Congress on Photosynthesis, CSIRO Publishing: S15–014.
Raghavendra A.S., Padmasree K., Saradadevi K. 1994. Interdependence of photosynthesis and respiration in plant cells — interactions between chloroplasts and mitochondria. Plant Sci., 97: 1–14.
Reddy M.M., Vani T., Raghavendra A.S. 1991. Light-enhanced dark respiration in mesophyll protoplasts from leaves of pea. Plant Physiol., 96: 1368–1371.
Ribas-Carbo M., Berry J.A., Azcón-Bieto J., Siedow J.N. 1994. The reaction of the plant mitochondrial cyanide-resistant alternative oxidase with oxygen. Biochim. Biophys. Acta, 1188: 205–212.
Romanowska E., Igamberdiev A.U., Parys E., Gardeström P. 2002. Stimulation of respiration by Pb2+ in detached leaves and mitochondria of C3 and C4 plants. Physiol. Plant., 116: 148–154.
Srinivasan R., Berndt W.A., Oliver D.J. 1993. Coordinated expression of photosynthetic and photorespiratory genes. In: Plant mitochondria, ed by A. Brennicke A, U. Kuck, VCH, Weinheim: 160–169.
Ta T.C., Joy K.W., Ireland R.J. 1985. Role of asparagines in the photorespiratory nitrogen metabolism of pea leaves. Plant Physiol., 78: 334–337.
Tobin A., Djerdjour B., Journet E., Neuburger M., Douce R. 1980. Effect of NAD+ on malate oxidation in intact plant mitochondria. Plant Physiol., 66: 334–337.
Vianello A., Braidot E., Petrussa E., Marci F. 1997. ATP synthesis driven by α-keto acid-stimulated alternative oxidase in pea leaf mitochondria. Plant Cell Physiol., 38: 1368–1374.
Wiskich J.T., Bryce J.H., Day D.A., Dry I.B. 1990. Evidence for metabolic domains within the matrix compartment of pea leaf mitochondria. Implications for photorespiratory metabolism. Plant Physiol., 93: 611–616.
Xue X., Gauthier D.A., Turpin D.H., Weger H.G. 1996. Interactions between photosynthesis and respiration in the green alga Chlamydomonas reinhardtii. Characterization of light-enhanced dark respiration. Plant Physiol., 112: 1005–1014.
Yamaya T., Oaks, A., Matsumoto H. 1984. Stimulation of mitochondrial calcium uptake by light during growth of corn shoots. Plant Physiol., 75: 733–777.
Zhang Q., Wiskich J.T. 1995. Activation of glycine decarboxylase in pea leaf mitochondria by ATP. Arch. Biochem. Biophys., 320: 250–256.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parys, E., Romanowska, E. & Siedlecka, M. Light-enhanced dark respiration in leaves and mesophyll protoplasts of pea in relation to photorespiration, respiration and some metabolites content. Acta Physiol Plant 26, 37–46 (2004). https://doi.org/10.1007/s11738-004-0042-7
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11738-004-0042-7