Abstract
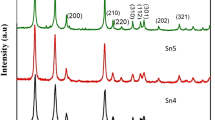
SnO2 and Sb-doped SnO2 particles were synthesized using the polymeric precursor method with different Sn salt precursors: SnCl2·2H2O, SnCl4·5H2O, or Sn citrate. Sb2O3 was used as the precursor of Sb, and the molar ratio of n Sn:n Sb was held constant. FTIR and TGA/DTA were used to examine the influence of the Sn precursor on the formation and thermal decomposition of the Sn and Sn-Sb complexes. The calcination products obtained from heating the Sn and Sn-Sb complexes at 500°C in air were analyzed using XRD and TEM analysis. The results revealed that the SnO2 and Sb-doped SnO2 formation temperatures depended on the nature of the Sn precursor. The calcination products were found to be SnO2 and Sb-doped SnO2 particles, which crystallized in a tetragonal cassiterite structure with a highly preferred (110) planar orientation. The Sn precursor and the presence of Sb in the SnO2 matrix strongly influenced the crystallinity and lattice parameters.
Similar content being viewed by others
References
Zhang H, He Q, Zhu X, et al. Surfactant free solution phase synthesis of monodispersed SnO2 hierarchical nanostructures and gas sensing properties. CrystEngComm, 2012, 14(9): 3169–3176
Wan Q, Wang T H. Single-crystalline Sb-doped SnO2 nanowires: synthesis and gas sensor application. Chemical Communications, 2005, 30(30): 3841–3843
Zum Felde U, Haase M, Weller H. Electrochromism of highly doped nanocrystalline SnO2:Sb. The Journal of Physical Chemistry B, 2000, 104(40): 9388–9395
Wang Y, Djerdj I, Smarsly B, et al. Antimony-doped SnO2 nanopowders with high crystallinity for lithium-ion battery electrode. Chemistry of Materials, 2009, 21(14): 3202–3209
Xu C H, Sun J, Gao L. Direct growth of monodisperse SnO2 nanorods on graphene as high capacity anode materials for lithium ion batteries. Journal of Materials Chemistry, 2012, 22(3): 975–979
Li Z D, Zhou Y, Yu T, et al. Unique Zn-doped SnO2 nano-echinus with excellent electron transport and light harvesting properties as photoanode materials for high performance dye-sensitized solar cell. CrystEngComm, 2012, 14(20): 6462–6468
Kim Y S, Yu B K, Kim D Y, et al. A hybridized electron-selective layer using Sb-doped SnO2 nanowires for efficient inverted polymer solar cells. Solar Energy Materials and Solar Cells, 2011, 95(10): 2874–2879
Lim J, Jeong B Y, Yoon H G, et al. Inkjet-printing of antimonydoped tin oxide (ATO) films for transparent conducting electrodes. Journal of Nanoscience and Nanotechnology, 2012, 12(2): 1675–1678
Leem J W, Yu J S. Physical properties of electrically conductive Sb-doped SnO2 transparent electrodes by thermal annealing dependent structural changes for photovoltaic applications. Materials Science and Engineering B, 2011, 176(15): 1207–1212
Wu S S, Cao H Q, Yin S F, et al. Amino acid-assisted hydrothermal synthesis and photocatalysis of SnO2 nanocrystals. Journal of Physical Chemistry C, 2009, 113(41): 17893–17898
Wang Y, Fan C, Hua B, et al. Photoelectrocatalytic activity of two antimony doped SnO2 films for oxidation of phenol pollutants. Transactions of Nonferrous Metals Society of China, 2009, 19(3): 778–783
Paniza M. Chapter 2: Importance of electrode material in the electrochemical treatment of wastewater containing organic pollutants. In: Comninellis C, Chen G, eds. Electrochemistry for the Environment. Springer, 2010, 25
Robertson J, Falabretti B. Chapter 2: electronic structure of transparent conducting oxides. In: Ginley D S, ed. Handbook of Transparent Conductors. New York: Springer, 2010, 27
Singh A K, Janotti A, Scheffler M, et al. Sources of electrical conductivity in SnO2. Physical Review Letters, 2008, 101(5):055502 (4 pages)
Li Z Q, Yin Y L, Liu X D, et al. Electronic structure and optical properties of Sb-doped SnO2. Journal of Applied Physics, 2009, 106(8): 083701
Al-Gaashani R, Radiman S, Tabet N, et al. Optical properties of SnO2 nanostructures prepared via one-step thermal decomposition of tin(II) chloride dihydrate. Materials Science and Engineering B, 2012, 177(6): 462–470
Yu D, Wang D, Yu W, et al. Synthesis of ITO nanowires and nanorods with corundum structure by a co-precipitation-anneal method. Materials Letters, 2004, 58(1–2): 84–87
Ibarguen C A, Mosquera A, Parra R, et al. Synthesis of SnO2 nanoparticles through the controlled precipitation route. Materials Chemistry and Physics, 2007, 101(2–3): 433–440
Nguyen T B, Le T T B, Nguyen N L. The preparation of SnO2 and SnO2:Sb nanopowders by a hydrothermal method. Advances in Natural Sciences: Nanoscience and Nanotechnology, 2010, 1(2):025002 (4 pages)
Korosi L, Papp S, Meynen V, et al. Preparation and characterization of SnO2 nanoparticles of enhanced thermal stability: The effect of phosphoric acid treatment on SnO2·nH2O. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2005, 268(1–3): 147–154
Seo M, Akutsu Y, Kagemoto H. Preparation and properties of Sbdoped SnO2/metal substrates by sol-gel and dip coating. Ceramics International, 2007, 33(4): 625–629
Zhu F L, Meng Y S. Synthesis and characterization of antimony doped tin oxide conductive nanoparticles by alkoxide hydrolysis method. Advanced Materials Research, 2013, 702: 167–171
Leite E R, Maciel A P, Weber I T, et al. Development of metal oxide nanoparticles with high stability against particle growth using a metastable solid solution. Advanced Materials, 2002, 14(12): 905–908
Rodrigues E C P E, Olivi P. Preparation and characterization of Sb-doped SnO2 films with controlled stoichiometry from polymeric precursors. Journal of Physics and Chemistry of Solids, 2003, 64(7): 1105–1112
Xu J M, Li L, Wang S, et al. Influence of Sb doping on the structural and optical properties of tin oxide nanocrystals. CrystEngComm, 2013, 15(17): 3296–3300
Zhong X, Yang B, Zhang X, et al. Effect of calcining temperature and time on the characteristics of Sb-doped SnO2 nanoparticles synthesized by the sol-gel method. Particuology, 2012, 10(3): 365–370
Aziz M, Saber Abbas S, Wan Baharom W R. Size-controlled synthesis of SnO2 nanoparticles by sol-gel method. Materials Letters, 2013, 91: 31–34
Jeng J S. The influence of annealing atmosphere on the material properties of sol-gel derived SnO2:Sb films before and after annealing. Applied Surface Science, 2012, 258(16): 5981–5986
Ningthoujam R S, Kulshreshtha S K. Nanocrystalline SnO2 from thermal decomposition of tin citrate crystal: luminescence and Raman studies. Materials Research Bulletin, 2009, 44(1): 57–62
Gordillo G, Moreno L C, de la Cruz W, et al. Preparation and characterization of SnO2 thin films deposited by spray pyrolysis from SnC12 and SnC14 precursors. Thin Solid Films, 1994, 252(1): 61–66
Comninellis Ch, Vercesi G P. Problems in DSA® coating deposition by termal decomposition. Journal of Applied Electrochemistry, 1991, 21(2): 136–142
Terrier C, Chatelon J P, Roger J A, et al. Analysis of antimony doping in tin oxide thin films obtained by the sol-gel method. Journal of Sol-Gel Science and Technology, 1997, 10(1): 75–81
Terrier C, Chatelon J P, Berjoan R, et al. Sb-doped SnO2, transparent conducting oxide from the sol-gel dip-coating technique. Thin Solid Films, 1995, 263(1): 37–41
Gonzalez-Oliver C J R, Kato I. Sn(Sb)-oxide sol-gel coatings on glass. Journal of Non-Crystalline Solids, 1986, 82(1–3): 400–410
Xu C, Xu G, Liu Y, et al. Preparation and characterization of SnO2 nanorods by thermal decomposition of SnC2O4 precursor. Scripta Materialia, 2002, 46(11): 789–794
Bhagwat M, Shah P, Ramaswamy V. Synthesis of nanocrystalline SnO2 powder by amorphous citrate route. Materials Letters, 2003, 57(9–10): 1604–1611
Pechini M P. US Patent, 3 330 697, 1967-07-01
Besso M M. US Patent, 3 213 120, 1965-10-19
Tselesh A S. Anodic behaviour of tin in citrate solutions: The IR and XPS study on the composition of the passive layer. Thin Solid Films, 2008, 516(18): 6253–6260
Chalupa J, Handlir K, Cisarova I, et al. Structural study of bis (triorganotin(IV)) esters of 4-ketopimelic acid. Journal of Organometallic Chemistry, 2006, 691(12): 2631–2640
Feng S, Tang Y, Xiao T. Anodization, precursor route to flowerlike patterns composed of nanoporous tin oxide nanostrips on tin substrate. Journal of Physical Chemistry C, 2009, 113(12): 4809–4813
Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc. Vol. 25, 2007
Batista P D, Mulato M, Graeff C F O, et al. SnO2 extended gate field-effect transistor as pH sensor. Brazilian Journal of Physics, 2006, 36(2a): 478–481
Grzeta B, Tkalcec E, Goebbert C, et al. Structural studies of nanocrystalline SnO2 doped with antimony: XRD and Mössbauer spectroscopy. Journal of Physics and Chemistry of Solids, 2002, 63(5): 765–772
Krishnakumar T, Jayaprakash R, Pinna N, et al. Structural, optical and electrical characterization of antimony-substituted tin oxide nanoparticles. Journal of Physics and Chemistry of Solids, 2009, 70(6): 993–999
Zhang D L, Tao L, Deng Z B, et al. Surface morphologies and properties of pure and antimony-doped tin oxide films derived by sol-gel dip-coating processing. Materials Chemistry and Physics, 2006, 100(2–3): 275–280
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
López Morales, F., Zayas, T., Contreras, O.E. et al. Effect of Sn precursor on the synthesis of SnO2 and Sb-doped SnO2 particles via polymeric precursor method. Front. Mater. Sci. 7, 387–395 (2013). https://doi.org/10.1007/s11706-013-0227-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11706-013-0227-3