Abstract
Nanomaterials have gained a lot of interest due to their application in various fields. Tin oxide (SnO2) is an important n-type wide band-gap semiconductor in the field of gas sensing devices due to its chemical and mechanical stability. Here, SnO2 nanoparticles were synthesized by a simple chemical co-precipitation method followed by annealing the obtained nanoparticles at different temperatures. Several characterization techniques like powder X-ray Diffraction, Scanning Electron Microscope, Energy-Dispersive X-Ray spectroscopy, and Ultraviolet–Visible spectroscopy, Fourier Transform Infrared Spectroscopy were carried out to analyze the structure, size, morphology, elemental composition and optical properties of the prepared SnO2 nanoparticles.
Similar content being viewed by others
1 Introduction
Nanomaterials have attracted the interest of scientists all across the globe after the famous inspiring speech by Feynman stating, ‘there is plenty of room at the bottom’ [1]. This ignited a revolution in the research community which led to exploration of materials and their properties down to the nano scale in every field of science. Nanoparticles of wide-bandgap semiconductors such as Tin Oxide (SnO2) have been in the limelight as they have found applications in gas sensing, humidity and temperature sensing, lithium batteries, transistors, transparent conducting electrodes, liquid crystal displays, photovoltaic devices and as a key element in opto-electronic devices [2,3,4,5,6,7,8,9]. The performance of such semiconducting nanoparticles like their response time, stability, selectivity, sensitivity etc. depends chiefly upon the size of particles, which in turn can be controlled by varying the pH of the solution, concentration of precursor and precipitating reagents [3, 4]. SnO2 is a versatile n-type semiconductor with a crystalline structure and depending on the synthesis procedure it can have optical (direct) transition corresponding to 3.6–3.8 eV or an indirect transition near 2.7–3.1 eV [5, 6].
SnO2 can be synthesized by various methods like crystal spray pyrolysis, hydrothermal methods, thermal evaporation, microwave-assisted combustion method, sol–gel method and electrochemical synthesis as mentioned in previous literatures [5,6,7,8,9,10,11,12,13]. Koshay et al. had prepared the nanoparticles through sol–gel technique having a tetragonal structure with an average particle size of 12.10 nm [2]. Naje et al. conducted experiments to obtain SnO2 nanoparticles through chemical precipitation method. The morphology shows tetragonal structure with particle size in the range of 8–10 nm [6]. Razeghizadeh et al. performed the experiments with Sol–Gel technique which results in tetragonal structure of SnO2 having different average crystallite size for different solution concentrations (17 nm, 18.8 nm, 74.8 nm for 0.1, 0.3, 0.5 M respectively) [7]. Thakre et al. made SnO2 thin solid films through physical vapour deposition technique. Further the thin films were used for gas sensing purpose [9]. Nehru et al. synthesized the SnO2 nanopowder through microwave assisted combustion method and tetragonal rutile structure with 8 nm particle size was found [12]. Chen et al. had synthesized the tin oxide nanoparticles through electrochemical synthesis having tetragonal rutile structure and the average size of the annealed particles was found to be 15.4, 12.5, 11.8 nm for different voltages i.e. 20 V, 30 V and 40 V respectively [14].
Amongst the various proposed methods, chemical co-precipitation method possesses the advantages of being easily reproducible, utilizes simple laboratory equipment, low in impurity, less time consuming, and ease of processability. Hence in this paper, chemical co-precipitation method has been chosen for synthesis of SnO2 nanoparticles (NPs) [15]. The prepared SnO2 NPs were annealed at three different temperatures. The finally obtained NPs were analyzed for their structure, size, morphology, elemental composition and optical properties by several characterization techniques like powder X-ray Diffraction (XRD), Scanning Electron Microscope (SEM), Energy-Dispersive X-Ray spectroscopy (EDX), Fourier Transform Infrared (FTIR) Spectroscopy, and Ultraviolet–Visible (UV) spectroscopy. XRD confirmed the tetragonal structure of the NPs and the average crystallite size increased with the rise in annealing temperature which led to change in the bandgap.
2 Materials
The chemicals were used as received without any additional purification. Stannous chloride (SnCl2⋅2H2O) was purchased from Central drug house (p) Ltd. having molecular weight 225.63 (99.6% pure). Ethanol was purchased from Changshu Hongsheng fine chemicals. Liquor Ammonia (NH3) and Hydrochloric acid (HCl) were purchased from Fisher Scientific. Double Distilled (DD) water prepared in laboratory was used wherever necessary.
3 Synthesis
SnO2 NPs were prepared by chemical co-precipitation method. 0.27 M SnCl2⋅2H2O was added to DD water and ethanol (2.2:1 v/v) in a 1000 mL beaker under continuous magnetic stirring for 60 min in order to obtain a homogeneous solution. 50 mL of HCl was added drop wise in order to reduce the precursor and the solution was kept on stirring for another 30 min to allow proper mixing resulting in a clear transparent solution. Later aqueous NH3was added dropwise till the pH of the solution became 8.5 and a milky white solution was obtained. The solution was kept on continuous stirring for another 3 h. After this the stirring was stopped and the beaker was kept undisturbed overnight in order to facilitate the particles to settle down completely.
The solution was then washed with DD water and ethanol multiple times so as to remove the residual chlorine content using 125 mm Whatman filter paper (Cat no. 1001 125). The resulting product was covered and left for drying overnight. The dried powder was then divided into three parts and annealed at 400 °C, 500 °C, and 600 °C named as Sn4, Sn5, and Sn6 respectively.
4 Characterization techniques
4.1 Sructure, morphology and elemental composition
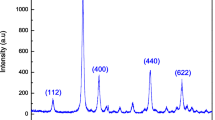
To identify the crystalline structure and crystallite size of the prepared NPs, XRD studies were carried out using X’Pert PRO Wide Angle X-Ray Diffraction (WAXD) using Cu-kα source with voltage 40 kV and current 30 mA in the 2θ range 20° to 90° at a scan speed of 2°/min. The obtained XRD pattern of Sn4, Sn5 and Sn6 is shown in Fig. 1. We can observe a consistent narrowing of the peaks as the annealing temperature was increased which reflects upon the decreasing full width at half maxima (FWHM) as we go from Sn4 to Sn6. The narrowing of peaks is directly related to the increase in average crystallite size of the NPs. With the increase in the annealing temperature, the SnO2 particles have more energy at their disposal which allows the particles to coalescence together, thereby leading to formation of bigger particles [16]. The crystalline size or average size of SnO2 NPs can be estimated by using Debye–Scherrer Formula as shown in Eq. (1).
where ‘D’ represents average crystallite size, ‘k’ is the shape factor having value 0.9, ‘λ’ is wavelength of Cu-kα radiation (1.54059 Ǻ), ‘β’ indicates FWHM of the peak and ‘θ’ is Bragg’s angle in degrees. The calculated average crystalline size of SnO2 NPs Sn4, Sn5 and Sn6 is 11.8 nm, 16.6 nm, and 35.8 nm respectively. Figure 2 shows the graphical representation of average crystallite size with the annealing temperature. The nonlinear increment in crystallite size with rise in annealing temperature is consistent with the available literature [17, 18].
The obtained XRD pattern corresponds to the tetragonal (rutile) phase of the SnO2 crystal structure as confirmed by the JCPDS data 41-1445 and 88-0287 [14, 19]. Table 1 summarizes the major peak position (2θ), the d-spacing that represents interplanar distance, miller indices, and FWHM of the three samples. It can be clearly seen that the FWHM decreases from Sn4 to Sn6 with an almost consistent d-spacing. Further, it can be observed that some small peaks are also present in all the three samples which represent different miller indices of the tetragonal phase of SnO2, however they tend to diminish in intensity with increase in average crystallite size. The absence of any overlapping peaks over the standard peaks confirms that the prepared NPs have single phase and are devoid of any impurities.
ZEISS EVO 50 SEM with an acceleration voltage of 15 kV and a magnification of 4 k X was used to check the surface morphology of the SnO2NPs.The powdered sample was spread uniformly on the conductive double-sided adhesive carbon tape (Micro to Nano, Product No. 15-000505). The obtained images are shown in Fig. 3. From the images it is clear that Sn4 (Fig. 3a) has almost circular shaped particles along with some non-uniform rod like structures and agglomerations. The non-uniformity in structure and agglomerations tend to increase in Sn5 and Sn6 (Fig. 3b, c). Owing to the increase in annealing temperature, the agglomerations in NPs occur leading to irregular morphology of the samples. As suggested by XRD, it is clearly observable through SEM that the average particle size has also increased from Sn4 to Sn6.
For assessing the elemental composition of the samples, RONTEC’s EDX system Model QuanTax 200 attached to the SEM instrument was used. The obtained figures for the three samples are shown in Fig. 4. Table 2 shows the weight % and the atomic % of the corresponding elements as obtained from Fig. 4. From the analysis it is clear that Sn and O peaks are present in majority in the appropriate ratio thereby confirming the formation of SnO2 NPs. Further trace peaks of C and Al can also be observed which are present due to the carbon tape that was used for the spreading of sample.
5 Optical study
5.1 UV–visible spectroscopy
Shimadzu 1800 UV–Vis spectrophotometer in the wavelength range of 200 nm to 800 nm was used to study the optical property of SnO2 NPs. Small amount of the samples Sn4, Sn5 and Sn6 were taken in sample vials with DD water and sonicated for 1 h so as to allow proper dispersion in the solvent. Few mL of sample was transferred to the quartz cuvette and dispersed in DD water for carrying out the analysis. The obtained absorption spectrum of Sn4, Sn5 and Sn6 NPs is shown in Fig. 5. The first absorption maxima (λmax) of Sn4, Sn5 and Sn6 is observed at 289 nm, 292 nm and 303 nm respectively. A secondary absorption peak can also be seen at ~ 230 nm which is consistent for all the three samples. The samples show a consistent rise right from 800 to ~ 400 nm after which change in slope is observed. This kind of behavior is typical with wide bandgap semiconductors since they show no absorption in the visible region [20].
The optical bandgap energy (Eg) was calculated by using Tauc’s relation a given in Eq. (2).
where ‘α’ is optical absorption coefficient, ‘h’ is planck’s constant, ‘ν’ is frequency of incident photon, ‘hν’ is energy of incident photon, ‘Eg’ is energy of optical bandgap. The plot (αhν)2 versus (hν) is obtained from it. Since the thickness of the sample is negligible in case of liquid medium, the value of ‘α’ is considered to be the same as that of Absorbance (A) which is obtained from the UV–Vis absorption. The value of optical band gap can be measured by extrapolating a straight line from the plot to intersect the horizontal photon energy axis (absorption equal to 0). As mentioned previously that SnO2 can possess both direct or indirect bandgap, however in our case it is direct bandgap as the indirect transition did not result in any ‘straight line’ which could be justified and extrapolated for evaluating the value of bandgap. From Fig. 6 it is observed that the optical bandgap energy of the nanoparticles does not follow the usual pattern of decrease in band gap with increase in the annealing temperature. The Eg of Sn4, Sn5 and Sn6 is found to be 3.5 eV, 3.7 eV, and 3.0 eV respectively. Compared to the direct bulk bandgap of SnO2 (~ 3.68 eV), as the annealing temperature increases the bandgap of NPs is found to decrease as well as increase. This happens due to the fact that SnO2 NPs do not follow quantum confinement effect strictly owing to the presence of defect sites in the crystal lattice and oxygen vacancies [21, 22]. The electronic structure of SnO2 has a complex architecture thus its bandgap never follows any defined pattern [23]. Owing to this, we can see that the bandgap of Sn5 has increased to 3.7 eV before decreasing to 3.0 eV in Sn6. This irregular change in bandgap can easily be correlated with the elemental stoichiometry obtained from the EDX analysis (Table 2). The O:Sn ratio in Sn5 is least compared to Sn4 and Sn6, hence we have obtained a deviation in bandgap of SnO2 NPs.
6 FTIR
The FTIR analysis of the SnO2 NPs was carried out using Agilent CARY 630 in the wavelength range 400–4000 cm−1 to identify the functional groups and confirm the formation of SnO2. Small amount of the NPs were sonicated in ethanol for an hour and 50 μL was dropped on the FTIR tip for analysis. The obtained spectrum is shown in Fig. 7. The broad peaks between 500 and 700 cm−1 indicates the formation of SnO2 [24, 25]. The rest of the peaks pertaining to C–H and O–H are due to the presence of ethanol thereby further confirming the purity of the prepared NPs. The obtained peaks are listed in Table 3.
7 Conclusion
In this paper we have successfully synthesized crystalline SnO2 NPs via chemical co-precipitation method. Since chemical co-precipitation method does not require any high-end instruments for synthesis and the method itself is free from any complications, hence the synthesized SnO2 NPs are low-cost and simple to reproduce. The XRD pattern revealed the tetragonal structure of the NPs with rutile phase. The average crystallite size of the NPs increased with increase in the annealing temperature while the optical bandgap energy showed deviation from the quantum confinement principle. SnO2 being a compound with complex electronic structure, the bandgap did not show any patterned change with annealing temperature. SEM revealed the agglomeration and non-uniform nature of the particles with increase in the annealing temperature which is consistent with our previous observations. The EDX analysis confirmed the preparation of SnO2 devoid of any impurities which was further affirmed with FTIR spectrum also. The chosen synthesis method is easily replicable for mass production of SnO2 NPs. Further, the small crystallite size of the SnO2 particles and the absence of any inherent impurities make it an excellent candidate for gas sensing purposes [26,27,28]. The prepared NPs are currently being tested for gas sensing applications and the results will be communicated shortly.
References
Feynman RP (1960) There's plenty of room at the bottom. California Institute of Technology, Engineering and Science magazine, Pasadena
Koshy J, Chandran A, Samuel S, George KC (2014) Optical properties of SnO2 nanoparticles. AIP Conf Proc 1620(1):192–196
Nahirniak S, Dontsova T, Astrelin I (2017) Directional synthesis of SnO2-based nanostructures for use in gas sensors. In: International conference on nanotechnology and nanomaterials. Springer, Cham, pp 233–245
Nahirniak SV, Dontsova TA, Chen Q (2018) Sensing properties of SnO2-MWCNTs nanocomposites towards H2. Mol Cryst Liq Cryst 674(1):48–58
Chand P (2019) Effect of pH values on the structural, optical and electrical properties of SnO2 nanostructures. Optik 181:768–778
Nadaf LI, Venkatesh KS (2016) Synthesis and characterization of tin oxide nanoparticles by co-precipitation method. Mater Sci 32:98–101
Naje AN, Norry AS, Suhail AM (2013) Preparation and characterization of SnO2 nanoparticles. Int J Innov Res Sci Eng Technol 2(12):7068–7072
Razeghizadeh AR, Zalaghi L, Kazeminezhad I, Rafee V (2015) Effects of Sol concentration on the structural and optical properties of SnO2 nanoparticle. arXiv preprint arXiv:abs/1502.00219
Diao GQ, Li H, Ivanenko I, Dontsova T, Astrelin I, Xie FY, Guo XZ (2019) Comparison of SnO2-carbon nanotubes composite and the SnO2-carbon black mixture as an anode for Li-ion batteries. In: IOP conference series: materials science and engineering, vol 474, no. 1. IOP Publishing, p 012022
Thakare KS, Patil SJ, Deshmukh SB, Borse RY, Ahire RR (2016) Preparation, characterization and gas sensing performance of pure SnO2 thin films deposited using physical vapour deposition. J Tech Eng 4(2):102–116
Chiu HC, Yeh CS (2007) Hydrothermal synthesis of SnO2 nanoparticles and their gas-sensing of alcohol. J Phys Chem C 111(20):7256–7259
Zhu JJ, Zhu JM, Liao XH, Fang JL, Zhou MG, Chen HY (2002) Rapid synthesis of nanocrystalline SnO2 powders by microwave heating method. Mater Lett 53(1–2):12–19
Nehru LC, Sanjeeviraja C (2014) Rapid synthesis of nanocrystalline SnO2 by a microwave-assisted combustsion method. J Adv Ceram 3(3):171–176
Chen W, Ghosh D, Chen S (2008) Large-scale electrochemical synthesis of SnO2 nanoparticles. J Mater Sci 43(15):5291–5299
Choudhury SP, Kumari N, Bhattacharjee A (2014) Comparative X-ray diffraction study of SnO2 nanoparticle samples from different precursor solutions. Cell 69:71–281
Gaber A, Abdel-Rahim MA, Abdel-Latief AY, Abdel-Salam MN (2014) Influence of calcination temperature on the structure and porosity of nanocrystalline SnO2 synthesized by a conventional precipitation method. Int J Electrochem Sci 9(1):81–95
Zhou W, Liu Y, Yang Y, Wu P (2014) Band gap engineering of SnO2 by epitaxial strain: experimental and theoretical investigations. J Phys Chem C 118(12):6448–6453
Kamble VB, Umarji AM (2013) Defect induced optical bandgap narrowing in undoped SnO2nanoparticles. AIP Adv 3(8):082120
Esmaeili-Bafghi-Karimabad A, Ghanbari D, Salavati-Niasari M, Nejati-Moghadam L, Gholamrezaei S (2015) Photo-catalyst tin dioxide: synthesis and characterization different morphologies of SnO2 nanostructures and nanocomposites. J Mater Sci 26(9):6970–6978
Paul Raj S (2013) Mineralization of azo dye using combined photo-fenton and photocatalytic processes under visible light. J Catal
Babar AR, Shinde SS, Moholkar AV, Rajpure KY (2010) Electrical and dielectric properties of co-precipitated nanocrystalline tin oxide. J Alloy Compd 505(2):743–749
Jain K, Lakshmikumar ST (2005) Preparation of nanocrystalline tin oxide powder for gas sensor applications. J Surf Sci Technol 21(3–4):129–138
Batzill M, Diebold U (2005) The surface and materials science of tin oxide. Prog Surf Sci 79(2–4):47–154
Aziz M, Abbas SS, Baharom WRW, Mahmud WZW (2012) Structure of SnO2 nanoparticles by sol–gel method. Mater Lett 74:62–64
Zinatloo-Ajabshir S, Morassaei MS, Salavati-Niasari M (2017) Facile fabrication of Dy2Sn2O7-SnO2 nanocomposites as an effective photocatalyst for degradation and removal of organic contaminants. J Colloid Interface Sci 497:298–308
Dieguez A, Romano-Rodriguez A, Morante JR, Weimar U, Schweizer-Berberich M, Göpel W (1996) Morphological analysis of nanocrystalline SnO2 for gas sensor applications. Sens Actuators B 31(1–2):1–8
Choudhary M, Mishra VN, Dwivedi R (2014) Gas sensing properties of tin oxide nano-powder synthesized via sol–gel route. In: Physics of semiconductor devices. Springer, Cham, pp 621–624
Zhang G, Liu M (2000) Effect of particle size and dopant on properties of SnO2-based gas sensors. Sens Actuators B 69(1–2):144–152
Acknowledgements
The authors are thankful to Central Research Facility, IIT-Delhi for helping us carry out the SEM and EDX studies, and to Dr. Manoj Kumar, Associate Prof., Jaypee Institute of Information Technology, Noida for the XRD and FTIR analysis. The author KS is thankful to Council of Scientific and Industrial Research (CSIR) India for granting the Senior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Naz, S., Javid, I., Konwar, S. et al. A simple low cost method for synthesis of SnO2 nanoparticles and its characterization. SN Appl. Sci. 2, 975 (2020). https://doi.org/10.1007/s42452-020-2812-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2812-2