Abstract
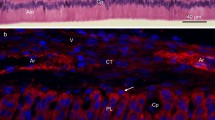
The fine structure of collar enamel and the cells constituting the enamel organ during amelogenesis in Lepisosteus oculatus was observed by light, scanning electron and transmission electron microscopy. In the enamel, slender crystals were arranged perpendicular to the surface and the stripes that were parallel to the surface were observed, suggesting that the enamel in Lepisosteus shares common morphological features with that in sarcopterygian fish and amphibians. Ameloblasts containing developed Golgi apparatus, rough endoplasmic reticulum (rER) and secretory granules were found in the secretory stage. In the maturation stage, a ruffled border was not seen at the distal end of the ameloblasts, while many mitochondria and lysosome-like granules were obvious in the distal cytoplasm. The enamel organ consisted of the outer dental epithelial cells, stratum reticulum cells and ameloblasts, but there was no stratum intermedium. It is likely that the ameloblasts have less absorptive function in comparison with the inner dental epithelial cells facing cap enameloid.
Similar content being viewed by others
References
Prostak K, Seifert P, Skobe Z. The effects of colchicine on the ultrastructure of odontogenic cells in the common skate, Raja erinacae. In: Fearnhead R W, ed. Tooth Enamel V. Yokohama, Japan: Florence, 1989, 188–192
Ishiyama M, Inage T, Shimokawa H. An immunocytochemical study of amelogenin proteins in the developing toothenamel of the gar-pike, Lepisosteus oculatus (Holostei, Actinopterygii). Archives of Histology and Cytology, 1999, 62(2): 191–197
Reif W-E. Evolution of dermal skeleton and dentition in vertebrates: the odontode regulation theory. Evolutionary Biology, 1982, 15: 287–368
Smith M M. Distribution and variation in enamel structure in the oral teeth of sarcopterygians: its significance for the evolution of a protoprismatic enamel. Historical Biology, 1989, 3: 97–126
Kerr T. Development and structure of some actinopterygian and urodele teeth. Proceedings of the Royal Society of London, Series B, Biological Sciences, 1960, 133: 401–422
Ishiyama M, Inage T, Shimokawa H. Abortive secretion of an enamel matrix in the inner enamel epithelial cells during an enameloid formation in the gar-pike, Lepisosteus oculatus (Holostei, Actinopterygii). Archives of Histology and Cytology, 2001, 64(1): 99–107
Sasagawa I, Ishiyama M. Fine structural and cytochemical mapping of enamel organ during the enameloid formation stages in gars, Lepisosteus oculatus, Actinopterygii. Archives of Oral Biology, 2005, 50(4): 373–391
Sasagawa I, Ishiyama M. Fine structural and cytochemical observations on the dental epithelial cells during cap enameloid formation stages in Polypterus senegalus, a bony fish (Actinopterygii). Connective Tissue Research, 2005, 46(1): 33–52
Shellis R P, Miles A E W. Autoradiographic study of the formation of enameloid and dentine matrices in teleost fishes using tritiated amino acids. Proceedings of the Royal Society of London, Series B, Biological Sciences, 1974, 185(1078): 51–72
Sasagawa I, Ishiyama M. The structure and development of the collar enameloid in two teleost fishes, Halichoeres poecilopterus and Pagrus major. Anatomy and Embryology, 1988, 178(6): 499–511
Isokawa S, Satomura I, Yamaguchi K, et al. Historadiographic observation on the outer dentine in certain osseus fishes. Journal of Nihon University School of Dentistry, 1970, 12: 1–5
Schmidt W J, Keil A. Polarizing Microscopy of Dental Tissues. Oxford: Pergamon Press, 1971, 584
Ørvig T. Microstructure and growth of the dermal skeleton in fossil actinopterygian fishes: Birgeria and Scanilepis. Zoological Scripta, 1978, 7: 33–56
Smith M M. Microstructure and evolution of enamel amongst osteichthyan fishes and early tetrapods. In: Smith P, Tchernov E, eds. Structure, Function and Evolution of Teeth. Proceedings of the 8th International Symposium on Dental Morphology. London: Freund Publishing House, 1992, 73–101
Reif W-E. Structural convergences between enameloid of actinopterygian teeth and of shark teeth. Scanning Electron Microscopy, 1979, II: 547–554
Peyer B. Comparative Odontology. Chicago: The University of Chicago Press, 1968, 321
Herold R C B. Ultrastructure of odontogenesis in the pike (Esox lucius). Role of dental epithelium and formation of enameloid layer. Journal of Ultrastructure Research, 1974, 48(3): 435–454
Shellis R P, Miles A E W. Observations with the electron microscope on enameloid formation in the common eel (Anguilla anguilla; Teleostei). Proceedings of the Royal Society of London, Series B, Biological Sciences, 1976, 194(1115): 253–269
Shellis R P, Poole D F G. The structure of the dental hard tissues of the coelacanthid fish Latimeria chalumnae Smith. Archives of Oral Biology, 1978, 23(12): 1105–1113
Smith M M. Enamel in the oral teeth of Latimeria chalumnae (Pisces: Actinistian): A scanning electron microscope study. Journal of Zoology (London), 1978, 185: 355–369
Sasagawa I, Ishiyama M, Kodera H. Fine structure of the pharyngeal teeth in the Coelacanthid fish (Latimeria chalumnae). In: Fearnhead R W, Suga S, eds. Tooth Enamel IV. Amsterdam: Elsevier, 1984, 462–466
Ishiyama M, Teraki Y. Microstructural features of dipnoan tooth enamel. Archives of Oral Biology, 1990, 35(6): 479–482
Kemp A. Ultrastructure of the developing dentition in the Australian lungfish, Neoceratodus forsteri. In: Smith P, Tchernov E, eds. Structure, Function and Evolution of Teeth. London: Freund Publishing House, 1992, 11–33
Kemp A. Ultrastructure of developing tooth plates in the Australian lungfish, Neoceratodus forsteri (Osteichthyes: Dipnoi). Tissue and Cell, 2003, 35(6): 401–426
Satchell P G, Shuler C F, Diekwisch T G H. True enamel covering in teeth of the Australian lungfish Neoceratodus forsteri. Cell and Tissue Research, 299(1): 27–37
Smith M M. Structure and histogenesis of tooth plates in Sagenodus inaequalis owen considered in relation to the phylogeny of post-devonian dipnoans. Proceedings of the Royal Society of London, Series B, Biological Sciences, 1979, 204(1154): 15–39
Smith M M, Hobdell M H, Miller W A. The structure of the scales of Latimeria chalumnae. Journal of Zoology (London), 1972, 167: 501–509
Zaki A E, MacRae E K. Fine structure of the secretory and nonsecretory ameloblasts in the frog. 1. Fine structure of the secretory ameloblasts. The American Journal of Anatomy, 1977, 148: 161–194
Zaki A E, MacRae E K. Fine structure of the secretory and non-secretory ameloblasts in the frog. II. Fine structure of the non-secretory ameloblast. Journal of Morphology, 1978, 158(2): 181–197
Zaki A E, Weber D F. Microradiography of the mineralization pattern in developing teeth of the frog, Rana pipiens. Archives of Oral Biology, 1979, 24(9): 651–655
Smith M M, Miles A E W. The ultrastucture of odontogenesis in larval and adult. urodeles; differentiation of the dental epithelial cells. Zeitschrift fur Zellforschung und mikroskopische Anatomie, 1971, 121: 470–498
Kogaya Y. Histochemical and immunohistochemical characterization of the ganoine layer of Polypterus senegalus. Association for Comparative Biology of Tooth Enamel, 1997, 4: 15–20
Kogaya Y. Immunohistochemical localisation of amelogenin-like proteins and type I collagen and histochemical demonstration of sulphated glycoconjugates in developing enameloid and enamel matrices of the larval urodele (Triturus pyrrhogaster) teeth. Journal of Anatomy, 1999, 195(3): 455–464
Chibon P. Etude ultrastructurale et autoradiographique des dents chez les amphibians. Relations entre la morphogenese dentaire et l’activite thyroidienne. Bulletin de la Societe Zoologique de France, 1972, 97: 437–448
Roux J P, Chibon P. Etude ultrastructurale de l’amelogenese chez la larve du triton Pleurodeles waltlii. [Amphibien Urodele]. Journal de Biologie Buccale, 1973, 1: 33–44
Kawasaki K, Fearnhead R W. Comparative histology of tooth enamel and enameloid. In: Suga S, ed. Mechanisms of Tooth Enamel Formation. Tokyo: Quintessence, 1983, 229–238
Bolte M, Clemen G. The enamel of larval and adult teeth of Ambystoma mexicanum shaw (Urodela: ambystomatidae)_a SEM study. Zoologischer Anzeiger, 1992, 228(3–4): 167–173
Wistuba J, Greven H, Clemen G. Development of larval and transformed teeth in Ambystoma mexicanum (Urodela, Amphibia): an ultrastructural study. Tissue and Cell, 2002, 34(1): 14–27
Davit-Béal T, Allizard F, Sire J-Y. Enameloid/enamel transition through successive tooth replacements in Pleurodeles waltl (Lissamphibia, Caudata). Cell and Tissue Research, 2007, 328(1): 167–183
Takagi J. The fine structure of salamander (Triturus pyrrhogaster) ameloblasts. Nihon University Dental Journal, 1991, 65, 10–18
Delgado S, Davit-Béal T, Allizard F, et al. Tooth development in a scincid lizard, Chalcides viridanus (Squamata), with particular attention to enamel formation. Cell and Tissue Research, 2005, 319(1): 71–89
Sire J-Y. Light and TEM study of nonregenerated and experimentally regenerated scales of Lepisosteus oculatus (holostei) with particular attention to ganoine formation. The Anatomical Record, 1994, 240(2): 189–207
Sire J-Y. Ganoine formation in the scales of primitive actinopterygian fishes, Lepisosteids and Polypterids. Connective Tissue Research, 1995, 33: 535–544
Kogaya Y. Histochemical and immunohistochemical characterization of the ganoine layer of Polypterus senegalus. Archives of Comparative Biology of Tooth Enamel, 1997, 5: 23–29
Zylberberg L, Sire J-Y, Nanci A. Immunodetection of amelogenin-like proteins in the ganoine of experimentally regenerating scales of calamoichthys calabaricus, a primitive actinoptery gian fish. The Anatomical Record, 1997, 249(1): 86–95
Sasagawa I, Ishiyama M. Fine Structure and Ca-ATPase activity of the stratum intermedium cells during odontogenesis in gars, Lepisosteus, Actinopterygii. Connective Tissue Research, 2002, 43(2 & 3): 505–508
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sasagawa, I., Ishiyama, M., Yokosuka, H. et al. Fine structure and development of the collar enamel in gars, Lepisosteus oculatus, Actinopterygii. Front. Mater. Sci. China 2, 134–142 (2008). https://doi.org/10.1007/s11706-008-0023-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11706-008-0023-7