Abstract
The preparation of zirconium dioxide nanoparticles (ZrO2-NPs) as hard ceramics was accomplished from rosette zircon concentrate through two consecutive alkaline digestion reactions. The rosette zircon concentration in the Abu Khashaba area consists mainly of zircon and monazite minerals. Using different operating conditions, the hydrothermal digestion by autoclave and the conventional alkaline fusion methods was performed upon the non-magnetic concentrate of rosette in order to complete the removal of monazite firstly and to complete the purification of zircon metal secondly. All monazite content and undesirable impurities were removed by the hydrothermal method using optimal digestion conditions such as 4 mol/L NaOH solutions, 1/6 solid to liquid, 2 h dissolving time, and a temperature of 423 K. The residual zircon (84% Zr) was subjected to complete digestion using NaOH with a zircon-to-alkali ratio of 1/1.5 and a fusion temperature of 923 K. ZrO2-NPs were synthesized using the hydrothermal technique at 473 K for 7 h. The calcined ZrO2-NPs were characterized by X-ray diffraction, scan electron microscope, and transmittance electron microscope. Purified silica was also obtained as a by-product from washing solutions of fused zircon.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zirconium is the eighteenth most plentiful element in the Earth's crust (Anderson 1989), as well as the eighth most abundant titanium group element, and has a concentration of about 130 mg/kg within the Earth's crust and about 0.026 μg/L in seawater (Attallah et al. 2021). The principal commercial source of zirconium is zircon (zirconium silicate ZrSiO4) (Perks and Mudd 2019). Zirconium can also be found as an impurity in a variety of minerals, including titanates, niobanates, tantalniobates, rare-earth silicates, and so on (Callaghan 2008; Bedinger 2017). Zirconium also occurs in more than 140 other minerals, including the commercially useful ores baddeleyite and eudialyte (Shahid et al. 2013). Globally, badelite and zircon are currently used in industry (Jolyon and Ralph 2008).
Because of its refractory nature, hardness, and resistance to chemical attack, zircon has many applications and is used directly in high-temperature applications such as ceramic materials and molds for molten metals (Manicone et al. 2007). Zirconium dioxide (ZrO2, also known as zirconia), which is the most common oxide produced from the mineral zircon, is used in the manufacture of laboratory crucibles, metallurgical furnaces, the production of ceramic knives, and as an ingredient in some abrasives (as grinding wheels and sandpaper) (Ali 2022; Lide 2008). Other niche applications for the zircon after conversion to metal were developed and used in many fields such as surgical appliances, light filaments, explosive primers, getters in vacuum tubes, and pyrotechnic compositions to generate sparks (Gudyanga 2020). Zirconium compounds had multi-uses such as using as adsorbents (Moradi et al. 2022). Zirconium was the main form used as a fuel cap for nuclear reactors consuming about 1% of the zirconium supply (Eliwa and Mubark 2021).
A variety of procedures have been investigated to extract zirconia from zircon, including alkaline digestion, chlorination, reductive smelting, and extraction with basic oxides. Carbohydrate chlorination is the most commonly used procedure as the decomposition of zircon requires high temperatures and harsh chemicals at all times. This was carried out by pumping the chlorine gas to the zircon and carbon mixture heated to roughly 1473 K (Nielsen and Wilfing 2010). Therefore, the alkaline fusion of zircon sand concentrates is highly efficient and can be used for large-scale production of ZrOCl2 economically (Xu and Xiao 2015). Alkaline fusion, also known as flux fusion, is widely employed in instead of acid dissolution. This type of dissolving is frequently used to dissolve materials such metal oxides, slags, and mineral ores that are resistant to acid assault. In order to perform flux fusion, a homogeneous mixture with an excessive amount of salt (flux) added to a mineral sample must be heated to a temperature higher than the flux salt’s melting point in a suitable vessel, such as a crucible (Abdel Wahab et al., 2022). The sample's ability to dissolve depends typically on the sample:flux weight ratio and the melting point of the flux salt. The melt is allowed to cool and is then dissolved in acid, base, or water after the reaction is determined to be complete visually by the disappearance of all the starting material.
In Egypt, large quantities of zircon are found in the rich deposits of black sand on the northern shores of the Nile Delta, from Rashid to Damietta (Osman et al. 2022). Monazite, a rare-earth mineral, is also abundant in these sand deposits (Abdel-Rehim 2005). Several techniques have been used to decompose monazite and recover the contents of the rare earth elements (REEs), thorium (Th), and uranium (U). Acid treatment and alkaline digestion were the most appropriate methods used (Singh 2007). Because of the refractory character of zircon and monazite, as well as their resistance to acid attack under moderate conditions, more aggressive leaching conditions were required (Biswas et al. 2010). Sintering with NaOH or KOH or a mixture of both at high temperatures was one of the commercially available methods (Eliwa et al. 2021; Borai et al. 2016). The main benefit of the alkaline approach was the separation of phosphorous in the form of sodium phosphate and the conversion of rare earth elements into hydroxides which facilitated their dissolution in dilute acids (El-Afandy et al. 2022).
Hydrothermal treatment is any heterogeneous reaction that occurs in the presence of aqueous solvents or metallic materials at high pressures and temperatures to melt or recrystallize materials that are generally intractable under normal conditions. Hydrothermal technology has also expanded into much broader fields that include many disciplines. As a new method, hydrothermal digestion technology has been developed in recent years to selectively extract one metal from the other (Naymanbaev 2009; Yang 2022; Al-Ghouti et al. 2021). This technology has an extraordinary reaction rate since it operates at a higher controllable temperature and pressure than conventional hydrometallurgical procedures.
Nano-materials have recently gained significant attention around the world due to their distinct physical and chemical properties as compared to their bulkier counterparts. To prepare materials at the nanoscale, several approaches have been devised such as co-precipitation (Abid et al. 2022; Zargari et al. 2014; Baig et al. 2021), electro-deposition (Selvam et al. 2013), sol–gel (Rahimi-Nasrabadi et al. 2013), mechano-chemical synthesis (Wu et al. 2007), microwave-assisted technique (Mancheva et al. 2011), a polymerized complex method (Kumar and Karuppuchamy 2015), hydrothermal (Ryu et al. 2003), and solvo-/hydrothermal technique (Arin et al. 2014; Subhan et al. 2021; Gul et al. 2019; Eliwa 2022).
Finding an efficient approach to fabricating nanocrystalline ZrO2 with particle sizes of a few nanometers was challenging. Thus, this work aims to prepare ZrO2-NPs using a simple, inexpensive, and friendly preparation technique. The current study was concerned with the method of preparation of ZrO2-NPs from the non-magnetic concentrate obtained from the Abu Khashaba beach area on the Mediterranean coast of Egypt using two distinct and consecutive methods. One was the hydrothermal digestion technique, and the other was the fusion digestion technique. The three main topics in this study were the selective decomposition of monazite leaving pure zircon mineral, complete digestion of zircon mineral using NaOH fluxing agent, and the hydrothermal preparation technique of ZrO2-NPs.
Experimental
Heavy fraction preparation and characterization
The ore sample was collected from the Abu Khashaba beach area on the Mediterranean coast, 7 km to the Rosetta estuary, Egypt. Several physical beneficiations methods such as shaking tables and magnetic separation were applied upon the ore sample to produce a concentrate as shown in Fig. 1 (Moustafa and Abdelfattah 2010). The resulting concentrate (heavy fraction) was considered the starting material for the experimental work of this study. The heavy fraction was firstly ground to the proper mesh size mesh before being subjected to digestion experiments.
The mineralogical composition of the heavy fraction of the Abu Khashaba sample was picked and investigated by a stereo microscope attached to an Olympus digital camera. Complete analysis of the heavy fraction components was performed using the traditional Shapiro and Brannock wet chemical technique (Shapiro and Brannock 1962). After complete dissolution of a representative sample of the heavy fraction, the major oxides such as SiO2 and P2O5 were determined using their relevant spectrophotometric methods while the total iron content was determined by titrimetric methods (Arin et al. 2014). Using the spectrophotometric method, the heavy fraction's rare earth and zirconium contents were measured at 650 and 535 nm, respectively (Marzenko 2000). Elemental analysis for Zr and REEs was performed using a double beam UV–VIS recording spectrophotometer Shimadzu UV160A.
Violet-colored complexes formed between Arsenazo III and rare earth elements were obtained at pH 2.3–2.7. The maximum absorption for these complexes was measured at 650 nm where the reagents do not absorb. On the other hand, xylenol orange reacted in acid medium with Zr ions to form a purple-red water-soluble complex, which has been recommended as a basis for determination of Zr and Hf (Michaylova et al. 1984). The most intense color between the Zr ions and the xylenol orange was obtained in 0.5–0.8 M HC1 media, and their absorbance was measured at 535 nm.
The metallic identification of the heavy fraction and as-prepared products was determined using a Philips PW 223/20 X-ray diffraction (XRD) operating at 40 kV and 20 mA with a Cu target. Ore morphology and the chemical composition of prepared products were studied by scanning electron microscopy (SEM–EDX 32). Mineral identification was accomplished using a Meiji-EMZ-TR stereo microscope model attached to an Olympus digital camera. The morphology of the synthesized zirconium oxide nanoparticles was determined and demonstrated using a transmission electron microscope (HTEM), Philips model 3M200.
All other chemicals were purchased from sigma: sodium hydroxide (NaOH, 99%), hydrochloric acid (HCl, 37%), ammonium hydroxide solution (NH4OH, 30%), and propyl alcohol (C3H8O, 99%). All chemicals and reagents used in this study were of analytical purity.
Hydrothermal digestion experiments
Heavy hydrothermal digestion experiments were performed using a homemade 50 mL autoclave as shown in Fig. 2. This was one of the most common autoclave designs similar to the general purpose autoclave and the Morey autoclave. A series of hydrothermal digestion experiments was performed using 5 g of Abu Khashaba concentrate and a sodium hydroxide solution. Digestion experiments were performed using various operating parameters such as NaOH concentration ranging from 2 to 8 mol/L, digestion temperature from 363 to 473 K, digestion time from 1 to 3 h, and solid-to-liquid ratio of 1/3 to 1/8. All the hydrothermal digestion experiments were performed three times to calculate the error bars for each experiment. After completing each hydrothermal experiment, the slurry was allowed to cool, filtered to separate the residue from the dissolved fraction, and washed with diluted HCL (5%). All chloride solutions obtained after hydrothermal treatments were analyzed to determine their components of REEs and Zr. To determine the dissolution efficiencies of these elements, the next equation (Eq. 1) was used.
During this study, the percentage of dissolution of rare earth elements served as a detector and evidence for the percentage of dissolution of their carrier metal, monazite, which is considered the main component of this mineral. Therefore, the results of REEs dissolution efficiencies were traced.
The obtained chloride solution was completely precipitated with NaOH. The product was filtered, washed, dried, and identified using EDX analysis. After optimum hydrothermal testing, the solid residue was washed with distilled water to remove chlorides and dried at 383 K for 6 h, and its components were determined using several instrumental analytical tools such as XRD, SEM, and EDX.
Zircon digestion experiments
Fusion digestion of solid residue was performed using sodium hydroxide as a fluxing agent. 5 g of the obtained solid residue after hydrothermal treatment was blended with 7.5 g of NaOH pellets, charged into a platinum crucible, and fused in an electric furnace at 623 K for 2 h. After cooling the crucible, the molten residue was firstly washed using hot distilled water and the residue was secondly dissolved using hot diluted hydrochloric acid (6 mol/L). Washing solutions were collected and treated with hydrochloric acid. The resulting gelatinous precipitate was filtered, dried, and analyzed with EDX to identify its components. The chloride filtrate solution was subjected to the subsequent experiments. The chloride filtration solution was subjected to subsequent experiments to synthesize zirconia nanoparticles.
Zirconia nanoparticles synthesis experiments
For the preparation of zirconia nanoparticles, an inexpensive technique was performed which is the hydrothermal technique. The ammonium hydroxide solution was added dropwise to the zirconium oxychloride (ZrOCl2) solution under ambient temperature (298 K ± 2) with constant stirring using a magnetic stirrer. The gelatinous precipitate formed at pH 2.4 was mixed with 30 mL of propyl alcohol, which acted as a surfactant, to reduce the surface tension. Finally, the mixture was transferred to a 50 ml Teflon-lined high-pressure autoclave and heated at 473 K for 7 h.
The precipitate was filtered using high-quality Whatman nanoporous filter paper and carefully rinsed with deionized water to remove residual chloride ions. The precipitate was then dried at 393 K for 24 h and calcined at 773 K for 2 h. Finally, the resulting white precipitate was described using various analytical techniques such as TEM, SEM, EDX, and XRD.
Results and discussion
Heavy fraction characterization results
The mineralization sample collected from Abu Khashaba area was subjected to different physical beneficiation steps such as desliming, wet tabling, and magnetic separation as illustrated in Fig. 1. The mineral composition of the non-magnetic fraction has been characterized using several techniques. Figure 3a, b illustrates the pictures of the picked minerals under a microscope. Zircon (ZrSiO4) and monazite (Ce,La,Nd,Th) (PO4,SiO4) minerals, which were the major minerals found in the non-magnetic concentrate, have been picked and photographed. The zircon minerals were characterized by varying colorless and pale brown shapes (Fig. 3b) while the monazite minerals were distinguished by deep canary and lemon yellow colors with spherical shapes elongated sub-rounded and well-rounded grains (Fig. 3a). Scanning electron microscopy (SEM) was also used to elucidate the morphology of the non-magnetic concentrate (Fig. 3c, d). Scanning the area for untreated mineral particles showed irregular edges and shapes, and some had flat faces.
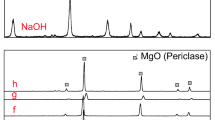
A complete elemental analysis of the non-magnetic concentration fraction was performed, and the chemical composition is presented in Table 1. A high proportion of SiO2 was detected, which was associated with the presence of the mineral zircon while the presence of P2O5 was related to the presence of the monazite mineral. It was evident from the results that the valuable constituents of interest were composed of 26% ZrO2, 18% RE2O3, 2.5% Fe2O3, 6.5% ThO2, and 2% U3O8. These results were identical to those obtained from EDX chart (Fig. 4a). From the XRD diagram, only zircon and monazite minerals appeared clearly as shown in Fig. 4b) (Abel Wahab et al., 2022; Liu et al. 2016; Udayakumar et al. 2020) which proved the presence of these two minerals unambiguously.
Hydrothermal digestion results
Effect of NaOH concentration
The alkali concentration plays an important role in the alkaline digestion technique. In most cases, the dissolution efficiency was gradually enhanced by increasing the alkali concentration, while sometimes this impeded the process. In this study, the effects of different NaOH concentrations (2–8 mol/L) on dissolution efficiencies of REEs and Zr were investigated.
From the results shown in Fig. 5a, the NaOH concentration has a significant effect on the dissolution efficiency of REEs without any effect on the dissolution of Zr. Dissolution efficiencies of 95, 97, 97, and 0.1% were accomplished for REEs and Zr, respectively, using a NaOH concentration of 4 mol/L. These promising results were achieved using constant conditions such as a reaction temperature of 423 K, a 1/6 solid/liquid ratio, and a reaction time of 2 h. Below 4 mol/L NaOH, the REEs elements had low dissolution efficiencies. By enhancing the alkaline concentration over 4 mol/L, the dissolution efficiency did not bring any improvements for the studied elements. So, the NaOH concentration of 4 mol/L was considered to be the optimum concentration.
Effect of digestion temperature
Since higher temperatures (over 373 K) not only improved mass transfer but also contributed to mineral dissolution and alteration, pressure filtration at higher temperatures was an effective approach to extract minerals from minerals (Shahid et al. 2013; Osman et al. 2022). To conduct a hydrothermal technique that selectively melts one mineral (monazite) without any effect on the other mineral (zircon), dissolution temperature must be controlled. Any increase in temperature above the appropriate temperature can cause the deterioration of zircon to begin. A range of dissolution temperatures from 363 to 473 K has been studied under other fixed conditions such as a NaOH concentration of 4 mol/L, a 1/6 solid/liquid ratio, and a reaction time of 2 h. Figure 5b shows the dissociation of REEs under varying temperatures. The dissolution efficiencies of REEs were improved by increasing the reaction temperature up to 423 K. There weren’t any enhancements in the recovery of the elements by increasing the temperature to 473 K. On the contrary, there was no significant effect on zircon mineral due to the increase in temperature. Therefore, 423 K was considered the optimum digestion temperature.
Effect of digestion time
To determine the optimal dissolution time at which maximum degradation of monazite can be achieved, reaction times ranging from 1 to 3 h were investigated using static conditions such as NaOH concentration of 4 mol/L, a 1/6 solid/liquid ratio, and a reaction temperature of 423 K. From the results shown in Fig. 5c, it turned out that the best dissolution time for REEs was 2 h achieving > 95% dissolution efficiency. By prolonging the time to more than 2 h, the dissolution efficiencies of all elements were not affected. Therefore, 2 h was the appropriate reaction time in which maximum dissolution of monazite occurred with minimum dissolution of zircon.
Effect of solid/liquid ratio
The effect of the solid/liquid ratio on the dissolution of REEs and Zr was investigated. These experiments were performed using a sodium hydroxide concentration of 4 mol/L, a reaction time of 2 h, and a reaction temperature of 423 K. Figure 5d shows the effect of changing the solid/liquid ratio from 1/3 to 1/6 under the previously mentioned conditions. By increasing the alkaline solution on the solid fraction from 1/3 to 1/6, the dissolution efficiencies of REEs were enhanced. These improvements have been associated with stability in the solubility of Zr. Very small improvements in the dissolution of REEs have occurred using the 1/8 solid/liquid ratio. As the solid/liquid ratio decreased, the total amount of NaOH solution increased, the mass transfer rate between the solid and alkali powder increased, and the dissolution kinetics increased [14]. Therefore, the solid/liquid ratio of 1/6 is the most appropriate.
By applying the optimum hydrothermal dissolution condition to the non-magnetic concentrate sample, the chloride feed solutions were collected and precipitated at pH 12.0 using NaOH solution (50%). The product was filtered, washed, dried, and identified using EDX analysis. From the EDX diagram shown in Fig. 6, impure REEs, Th, and U precipitate was detected which will undergo further purification steps in the future. The acquired precipitate contains large amounts of the studied elements with other contaminants resulting from direct precipitation with sodium hydroxide.
Zircon digestion results
The residue remaining after hydrothermal digestion was characterized using SEM, XRD, and EDX techniques. As shown in Fig. 7a,b, the morphology of the residues has colorless to brown irregular cylindrical shapes similar to that of zircon in Fig. 3b,c. These results were also corroborated by the XRD plot of the residues presented in Fig. 7c and its EDX scheme in Fig. 7d. Only zircon mineral was identified in the XRD diagram while monazite did not appear. EDX analysis proved the chemical composition of zircon mineral without any contamination from REEs, Th, or U from monazite. All these results prove the superiority of using hydrothermal digestion technology in removing other minerals present in the non-magnetic core leaving a residue of pure zircon.
The remained zircon was undergoes to complete fusion digestion using NaOH as a flux. Generally, zircon was hydrolyzed by fusion digestion with sodium hydroxide resulting in the formation of sodium zirconate and sodium silicate as follows (Eq. 2) (Sinha 1992). Sodium flakes were mixed with zircon mineral in a 1.5/1 alkali/mineral ratio and fused for 2 h at 923 K.
After cooling, the molten zircon was washed several times with water; the sodium zirconate was hydrolyzed to generate hydrated zirconia and sodium silicate that could be eliminated (Eq. 3).
Acid digestion of the hydrated zirconia was performed using concentrated hydrochloric acid (10 mol/L) under conditions of a 4:1 acid-to-hydrated zirconia ratio (v/w) and agitation for approximately 1 h at 373 K. The slurry was allowed to age at ambient temperature for 4 days. After aging, the slurry was filtered and the residue was washed with concentrated hydrochloric acid. The residue was then filtered with demineralized (DM) water, and the slurry was kept for one day for further aging. Finally, all filtrate solutions, containing zirconium oxychloride species, were collected and left a white solid residue. The separation of the residual silica from the soluble zirconium complex was achieved using an HCl solution according to Eq. 4. It was also obtained from the neutralization of a solution of sodium silicate with HCl as illustrated in Eq. 5.
The obtained white precipitate (from Eqs. 4 and 5) was collected, washed several times with hot distilled water to remove NaCl, dried at 393 K to remove hydration water, and labeled with EDX as shown in Fig. 7e. Pure silica has been obtained as a by-product of the alkaline digestion process that can be used in many other applications (Dhmees et al. 2019).
Synthesis of zirconia nanoparticles
Zirconium oxychloride feed solutions were collected and subjected to selective precipitation of gelatinous zirconia using ammonia. The feed solution was set at pH 2.4 to prevent iron precipitation. The reaction between ammonium hydroxide and zirconium chloride oxide is illustrated in Eq. 6.
The gelatinous precipitate was mixed with the organic surfactant, propyl alcohol, with a 10/1 gelatinous precipitate-to-surfactant ratio (v/v) and charged in an autoclave for hydrothermal treatment. The mixture was digested at 473 K for 7 h, and then, the product was filtered, rinsed, dried, calcined, and a white precipitate was produced. A complete analysis of the white precipitate was performed using XRD, SEM, EDX, and TEM to determine the chemical composition and elucidate the morphology as shown in Fig. 8.
From the SEM and TEM micrographs shown in Fig. 8a–f, the calcined ZrO2 nanoparticles had small monoclinic crystals and they agglomerated due to the nanocrystal size and the proximity of the particles. Very small particles were detected in a TEM micrograph that demonstrates the nanostructure of ZrO2. Sharp peaks were detected at [111], [200], and [220] planes in the XRD scheme as shown in Fig. 8g, proving the formation of zirconia. XRD peaks were used to estimate the size of ZrO2 crystallites. The crystallite size was determined from the diffraction peaks using Scherrer's equation (Eq. 6). By calculations, it was found that it was in the range of 3 to 15 nm. The EDX scheme of nanoparticles has demonstrated that the main components of ZrO2 precipitate were Zr with a purity of 93.73% and with shallow impurities of 2.37% Si and 3.9% Al as shown in Fig. 8h.
The processing stages of the non-magnetic Abu Khashaba concentrate to produce ZrO2-NPs were summarized in a flow sheet presented in Fig. 9.
Conclusion
Two alkaline treatments were carried out on a non-magnetic concentrate obtained from the Abu Khashaba beach area on the Mediterranean coast of Egypt. This concentrate consisted mainly of the minerals zircon and monazite with traces of other accessory minerals. Hydrothermal digestion and conventional fusion digestion were performed using NaOH one by one on the non-magnetic concentrate. Monazite and other contaminated mineral contents were completely hydrolyzed using hydrothermal treatment yielding pure zircon. Second, the complete decomposition of zircon minerals was achieved using conventional fusion digestions that yield zirconium oxychloride feed solutions. Hydrothermal treatment was re-used a second time for the selective deposition of zirconia in the nanoscale. Optimum operating conditions were performed on a zirconium oxychloride solution to produce zirconia nanocrystalline powder with crystal sizes from 3 to 15 nm. The morphological and the chemical composition of ZrO2-NPs were determined and confirmed using SEM, EDX, XRD and TEM analysis techniques. Therefore, the hydrothermal technique has succeeded in the simple, inexpensive, and facile treatment of the non-magnetic concentration that resulted in ZrO2-NPs.
References
Abdel-Rehim AM (2005) A new technique for extracting zirconium form Egyptian zircon concentrate. Int J Miner Process 76:234–243
Abdel Wahab GM, Abdellah WM, Yousif AM, Mubark AE (2022) Preparation of Pure Nb2O5 from Gabal El-Faliq Pegmatite, South Eastern Desert, Egypt. Min Metall Explor 39:833. https://doi.org/10.1007/s42461-019-00136-1
Al-Ghouti MA, Khan M, Nasser MS, Al Saad K, Heng OE (2021) A novel method for metals extraction from municipal solid waste using a microwave-assisted acid extraction. J Clean Prod 287(10):125039. https://doi.org/10.1016/j.jclepro.2020.125039
Abid N, Khan AM, Shujait S, Chaudhary K, Ikram M, Imran M, Haider J, Khan M, Khan Q, Maqbool M (2022) Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: a review. Adv Colloid Interface Sci 300:102597. https://doi.org/10.1016/j.cis.2021.102597
Ali AH (2022) Production of highly purifed zirconium oxide from zircon mineral leach liquor using bis-(2-ethylhexyl) phosphate as a promising extractant. Chem Pap. https://doi.org/10.1007/s11696-022-02339-1
Anderson DL (1989) Theory of the Earth. Blackwell Scientific Publications, Boston
Arin J, Dumrongrojthanath P, Yayapao O, Phuruangrat A, Thongtem S, Thongtem T (2014) Synthesis, characterization and optical activity of La-doped ZnWO4 nanorods by hydrothermal method. Superlattices Microstruct 67:197–206. https://doi.org/10.1016/j.spmi.2013.12.024
Attallah MF, El Afifi EM, Abdelsamad AA, Rizk HE, Massoud A (2021) Extraction chromatography and fractional precipitation procedures for production various zirconium grades for industrial and nuclear interest from Egyptian zircon ore. Arab J Nucl Sci Appl 54(2):63–72
Baig N, Kammakakam I, Falath W (2021) Nanomaterials: a review of synthesis methods, properties, recent progress, and challenges. Mater Adv 2:1821–1871. https://doi.org/10.1039/D0MA00807A
Bedinger GM (2017) Zirconium and Hafnium—2017 [Advance Release]. U.S. Geological Survey Minerals Yearbook—2017. file:///D:/Hayat%20zirconium%20paper/myb1–2017-zirco.pdf
Biswas RK, Habib MA, Karmakar AK, Islam MR (2010) A novel method for processing of Bangladeshi zircon: part I: baking, and fusion with NaOH. Hydrometallurgy 103(1–4):124–129
Borai EH, Abd El-Ghany MS, Ahmed IM, Hamed MM, Shah El-Din AM, Aly HF (2016) Modified acidic leaching for selective separation of thorium, phosphate and rare earth concentrates from Egyptian crude monazite. Int J Min Process 149:34–41. https://doi.org/10.1016/j.minpro.2016.02.003
Callaghan R (2008) Zirconium and Hafnium statistics and information. US Geological Survey. http://minerals.usgs.gov/minerals/pubs/commodity/zirconium/
Dhmees AS, Rashad AM, Eliwa AA, Zawrah MF (2019) Preparation and characterization of nano SiO2@CeO2 extracted from blast furnace slag and uranium extraction waste for wastewater treatment. Ceram Int 45:7309–7317
El-Afandy AH, Yousif AM, Mubark AE (2022) Subsequent separation of niobium (Nb), thorium (Th), rare earth elements (REEs), zirconium (Zr), and uranium (U) from Abu Rusheid cataclastic concentrate. South East Desert Radiochem 64(2):257–267. https://doi.org/10.1134/S1066362222020175
Eliwa AA (2022) Potentiality of using synthesized zinc tungstate nanoparticles for thoron and arsenazo III removal from waste solutions. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2022.2116980
Eliwa AA, Gawad EA, Mubark AE, Abel-Fattah NA (2021) Intensive studies for modeling and thermodynamics of fusion digestion processes of abu rusheid mylonite rocks. JOM 73:3419–3429. https://doi.org/10.1007/s11837-021-04837-1
Eliwa AA, Mubark A (2021) Effective sorption of U(VI) from chloride solutions using zirconium silico-tungstate matrix. Int J Env Anal Chem. https://doi.org/10.1080/03067319.2021.1921762
Gudyanga FP (2020) Minerals in Africa: opportunities for the continent’s industrialization. CRC Press, Taylor and Francais group, London, UK
Gul S, Khan SB, Rehman IU, Khan MA, Khan MI (2019) A comprehensive review of magnetic nanomaterials modern day theranostics. Front Mater 6:179. https://doi.org/10.3389/fmats.2019.00179
Jolyon R, Ralph I (2008) Minerals that include Zr. Mindat.org. http://www.mindat.org/chemsearch.php?inc=Zr%2C&exc=&sub=Search+for+Minerals
Kumar RD, Karuppuchamy S (2015) Synthesis and characterization of nanostructured Zn-WO3 and ZnWO4 by simple solution growth technique. J Mater Sci Mater in Electron 26:3256–3261. https://doi.org/10.1007/s10854-015-2824-7
Lide DR (2008) Zirconium: CRC handbook of chemistry and physics. CRC Press, New York
Liu J, Song J, Qi T, Zhang C, Qu J (2016) Controlling the formation of Na2ZrSiO5 in alkali fusion process for zirconium oxychloride production. Adv Powder Technol 27(1):1–8. https://doi.org/10.1016/j.apt.2015.08.005
Mancheva M, Iordanova R, Dimitriev Y (2011) Mechanochemical synthesis of nanocrystalline ZnWO4 at room temperature. J Alloy Compd 509:15–20. https://doi.org/10.1016/j.jallcom.2010.08.033
Manicone PF, Raffaelli Rossi P, IL, (2007) An overview of zirconia ceramics: basic properties and clinical applications. J Dent 35(11):819–826. https://doi.org/10.1016/j.jdent.2007.07
Marzenko Z (2000) separation and spectrophotometric determination of elements. Ellis Horwood, Chichester, England
Michaylova V, Šůcha L, Suchánek M (1984) Purification of xylenol orange by preparative paper chromatography, and examination of its zirconium complex. Talanta 31(8):645–647. https://doi.org/10.1016/0039-9140(84)80190-3
Moradi O, Panahandeh S (2022) Fabrication of different adsorbents based on zirconium oxide, graphene oxide, and dextrin for removal of green malachite dye from aqueous solutions. Environ Res 214(4):114042. https://doi.org/10.1016/j.envres.2022.114042
Moustafa MI, Abdelfattah NA (2010) physical and chemical beneficiation of the Egyptian beach monazite. Resour Geol 60(3):288–299
Naymanbaev AG (2009) Modern state of the technology of production of rare earth elements: socio-political literature. Kazakhstan Min J 2:24–25
Nielsen R, Wilfing G (2010) Zirconium and zirconium compounds: Ullmann's encyclopedia of industrial chemistry. Wiley Online. https://doi.org/10.1002/14356007.a28_543.pub2
Osman AM, Seif RA, Attia RM, El-Azab A, Khalifa IH (2022) Radiological and mineralogical studies on the coastal area between Port-Said and Damietta. J Phys Conf Ser 2305(1):012030
Perks C, Mudd G (2019) Titanium, zirconium resources and production: a state of the art literature review. Ore Geol Rev 107:629–646. https://doi.org/10.1016/j.oregeorev.2019.02.025
Rahimi-Nasrabadi M, Pourmortazavi SM, Ganjali MR, Hajimirsadeghi SS, Zahedi MM (2013) Electrosynthesis and characterization of zinc tungstate nanoparticles. J Mol Struct 1047(31):31–36. https://doi.org/10.1016/j.molstruc.2013.04.050
Ryu JH, Lim CS, Auh KH (2003) Synthesis of ZnWO4 nanocrystalline powders, by the polymerized complex method. Mater Lett 57:1550–1554. https://doi.org/10.1016/S0167-577X(02)01022-4
Selvam CS, Manikandan A, Kennedy LJ, Vijaya JJ (2013) Comparative investigation of zirconium oxide (ZrO2) nano and microstructures for structural, optical and photocatalytic properties. J Colloid Interface Sci 389:91–98
Shahid M, Ferrand E, Schreck E, Dumat C (2013) Behavior and impact of zirconium in the soil-plant system: plant uptake and phytotoxicity. Rev Environ Contam Toxicol 221:107–127. https://doi.org/10.1007/978-1-4614-4448-0_2
Shapiro L, Brannock NW (1962) Rapid analysis of silicate, carbonate and phosphate. U S Govt Print Off, Washington. https://doi.org/10.3133/b1144A
Singh G (2007) Chemistry of D-Block elements. Discovery Publish House, India, pp 315–320
Sinha HN (1992) From zircon to high purity zirconia for ceramics. Miner Process Extr Metall Rev 9(1–4):313–325
Subhan MA, Choudhury KP, Neogi N (2021) Advances with molecular nanomaterials in industrial manufacturing applications. Nanomanufacturing 1:75–97. https://doi.org/10.3390/nanomanufacturing1020008
Udayakumar S, Mohd Noor AF, Sheikh Abdul Hamid SAR, Putra TAR, Anderson CG (2020) Chemical and mineralogical characterization of Malaysian monazite concentrate. Min Metall Explor 37:415–431. https://doi.org/10.1007/s42461-019-00173-w
Wu Y, Zhang SC, Zhang LW, Zhu YF (2007) Photocatalytic activity of nanosized ZnWO4 prepared by the sol-gel method. Chem Res Chin Univ 23(4):465–468. https://doi.org/10.1016/S1005-9040(07)60100-7
Xu L, Xiao Y, Sandwijk A, Xu Q, Yang Y (2015) Production of nuclear grade zirconium: a review. J Nucl Mater 466:21–28
Yang J, Sun H, Peng T, Zeng L, Zhou X (2022) Mild hydrothermal synthesis of 11Å-TA from alumina extracted coal fly ash and its application in water adsorption of heavy metal ions (Cu(II) and Pb(II)). Int J Environ Res Public Health 19(2):616. https://doi.org/10.3390/ijerph19020616
Zargari S, Berijani MY, Rahimi R (2014) Synthesis of ZnO nanorods via coprecipitation method and its sensitizing with tetrakis (4-carboxy phenyl) porphyrin and its tin complex to enhance the visible light photocatalytic activity. J Nanomater 4(19):161
Acknowledgements
The authors would like to thank the collaborating members of the Nuclear Materials Authority for their assistance and cooperation in completing this work.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There were no potential conflicts of interest revealed by the authors. The authors confirm that there are no relevant financial or non-financial competing interests to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El Agamy, H.H., Mubark, A.E., Gamil, E.A. et al. Preparation of zirconium oxide nanoparticles from rosette concentrate using two distinct and sequential techniques: hydrothermal and fusion digestion. Chem. Pap. 77, 3229–3240 (2023). https://doi.org/10.1007/s11696-023-02699-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-02699-2