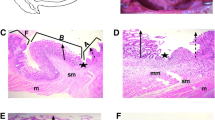
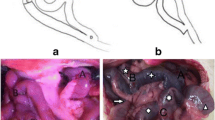
Abstract
Background
Gastric bypass with a proximal gastric pouch (Roux-en-Y gastric bypass) induces early diabetes remission. The effect of gastric bypass with a distal gastric pouch remains unknown.
Objective
To observe the effect on glucose tolerance and diabetes remission of gastric bypass with a distal gastric pouch.
Method
A type 2 diabetes (T2D) model was created in 44 Sprague-Dawley (SD) rats that randomly underwent Roux-en-Y gastric bypass (RYGB, n = 8); gastric bypass with duodenal-jejunal transit (GB-DJT, n = 8); distal-pouch gastric bypass with duodenal-jejunal transit (DPGB-DJT, n = 8); distal-pouch gastric bypass with duodenal-jejunal bypass (DPGB-DJB, n = 8); sham (n = 6); and Roux-en-Y gastric bypass with esophageal re-anastomosis (RYGB-Er, n = 6) surgery. In the DPGB-DJT and the DPGB-DJB groups, the gastric pouch was created in the distal stomach. In the RYGB and the GB-DJT groups, the gastric pouch was created in the proximal stomach. An oral glucose tolerance test (OGTT), insulin tolerance test (ITT) and mixed-meal tolerance test (MMTT) conducted preoperatively were repeated postoperatively.
Results
GLP-1 AUC recorded preoperatively was significantly increased 8 weeks postoperatively in the RYGB, GB-DJT, and DPGB-DJB groups. Increased GLP-1 AUC in the DPGB-DJT did not reach statistical significance. Improved glucose tolerance in the RYGB and GB-DJT groups was significantly higher than DPGB-DJT group. DPGB-DJB did not improve glucose tolerance significantly. Gastrin level was increased significantly in the DPGB-DJT and DPGB-DJB groups.
Conclusion
In gastric bypass, creating the gastric pouch in the distal region of the stomach significantly impairs the glucose tolerance and diabetes remission in spite of the increased GLP-1 and insulin responses in T2D SD rat model, suggesting that bypassing the distal stomach may be the key mediator of early diabetes remission after RYGB.





Similar content being viewed by others
References
Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98.
Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med. 2014;370(21):2002–13.
Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376(7):641–51.
Wickremesekera K, Miller G, Naotunne TD, et al. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg. 2005;15(4):474–81.
Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004;239(1):1–11.
Li P, Zhu L, Wang G, et al. The role of foregut exclusion in the deterioration of glucose and lipid metabolism induced by a high-fat diet. Diabetes Res Clin Pract. 2016;114:83–92.
Zervos EE, Agle SC, Warren AJ, et al. Amelioration of insulin requirement in patients undergoing duodenal bypass for reasons other than obesity implicates foregut factors in the pathophysiology of type II diabetes. J Am Coll Surg. 2010;210(5):564–72. 572-564
Kim DJ, Paik KY, Kim MK, et al. Three-year result of efficacy for type 2 diabetes mellitus control between laparoscopic duodenojejunal bypass compared with laparoscopic Roux-en-Y gastric bypass. Ann Surg Treat Res. 2017;93(5):260–5.
Heo Y, Ahn JH, Shin SH, et al. The effect of duodenojejunal bypass for type 2 diabetes mellitus patients below body mass index 25 kg/m(2): one year follow-up. J Korean Surg Soc. 2013;85(3):109–15.
Shimizu H, Eldar S, Heneghan HM, et al. The effect of selective gut stimulation on glucose metabolism after gastric bypass in the Zucker diabetic fatty rat model. Surg Obes Relat Dis. 2014;10(1):29–35.
Jirapinyo P, Thompson AC, Kroner PT, Chan WW, Thompson CC: Metabolic Effect of Foregut Exclusion Demonstrated by the Impact of Gastrogastric Fistula on Recurrence of Diabetes. J Am Coll Surg 2018, 226(3):259–266 e251.
Dolo PR, Yao L, Li C, Zhu X, Shi L, Widjaja J: Preserving Duodenal-Jejunal (Foregut) Transit Does Not Impair Glucose Tolerance and Diabetes Remission Following Gastric Bypass in Type 2 Diabetes Sprague-Dawley Rat Model. Obes Surg 2018, 28(5):1313–1320.
Zhang M, Lv XY, Li J, et al. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Exp Diabetes Res. 2008;2008:704045.
Allen RE, Hughes TD, Ng JL, et al. Mechanisms behind the immediate effects of roux-en-Y gastric bypass surgery on type 2 diabetes. Theor Biol Med Model. 2013;10:45.
Rubino F, Gagner M, Gentileschi P, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240(2):236–42.
Santoro S, Castro LC, Velhote MC, et al. Sleeve gastrectomy with transit bipartition: a potent intervention for metabolic syndrome and obesity. Ann Surg. 2012;256(1):104–10.
Reinehr T, Roth CL. The gut sensor as regulator of body weight. Endocrine. 2015;49(1):35–50.
Breen DM, Rasmussen BA, Kokorovic A, et al. Jejunal nutrient sensing is required for duodenal-jejunal bypass surgery to rapidly lower glucose concentrations in uncontrolled diabetes. Nat Med. 2012;18(6):950–5.
Geloneze B, Geloneze SR, Chaim E, et al. Metabolic surgery for non-obese type 2 diabetes: incretins, adipocytokines, and insulin secretion/resistance changes in a 1-year interventional clinical controlled study. Ann Surg. 2012;256(1):72–8.
Patel RT, Shukla AP, Ahn SM, et al. Surgical control of obesity and diabetes: the role of intestinal vs. gastric mechanisms in the regulation of body weight and glucose homeostasis. Obesity (Silver Spring). 2014;22(1):159–69.
Rubino F, R'Bibo SL, del Genio F, et al. Metabolic surgery: the role of the gastrointestinal tract in diabetes mellitus. Nat Rev Endocrinol. 2010;6(2):102–9.
Grong E, Graeslie H, Munkvold B, et al. Gastrin secretion after bariatric surgery-response to a protein-rich mixed meal following Roux-en-Y gastric bypass and sleeve gastrectomy: a pilot study in normoglycemic women. Obes Surg. 2016;26(7):1448–56.
Stenstrom B, Zhao CM, Tommeras K, et al. Is gastrin partially responsible for body weight reduction after gastric bypass? Eur Surg Res. 2006;38(2):94–101.
Zhou D, Jiang X, Jian W, et al. Comparing the effectiveness of total gastrectomy and gastric bypass on glucose metabolism in diabetic rats. Obes Surg. 2016;26(1):119–25.
Theodorakis MJ, Carlson O, Michopoulos S, et al. Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am J Physiol Endocrinol Metab. 2006;290(3):E550–9.
Ramracheya RD, McCulloch LJ, Clark A, et al. PYY-dependent restoration of impaired insulin and glucagon secretion in type 2 diabetes following Roux-En-Y gastric bypass surgery. Cell Rep. 2016;15(5):944–50.
Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest. 2012;122(1):4–12.
Tharakan G, Behary P, Wewer Albrechtsen NJ, et al. Roles of increased glycaemic variability, GLP-1 and glucagon in hypoglycaemia after Roux-en-Y gastric bypass. Eur J Endocrinol. 2017;177(6):455–64.
Funding
This study is supported by the Natural Science Foundation of Jiangsu Province (no. 2015102015).
Author information
Authors and Affiliations
Contributions
Ponnie Robertlee Dolo (ponnie85@yahoo.com) and Xiaocheng Zhu (zhuxccf@163.com) designed the experiment. Ponnie Robertlee Dolo performed the experiment and drafted the Manuscript. Chao Li (18068718716@189.cn) and Yong Shao (shaoyutong1975@163.com) supervised the experiment and offered technical support. Libin Yao (yaolibin_123@163.com) and Xiaocheng Zhu, revised the manuscript. Hui Wang (15996960499@139.com) Provided Material and logistical support.
Corresponding author
Ethics declarations
The study was approved by the ethics committee of Xuzhou Medical University Research Animal Center.
Conflict of Interest
The authors declare that they have no conflict of interest.
Statement of Animal Rights
All applicable institutional and national guidelines of the People’s Republic of China for the care and use of animals were followed.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dolo, P.R., Shao, Y., Li, C. et al. The Effect of Gastric Bypass with a Distal Gastric Pouch on Glucose Tolerance and Diabetes Remission in Type 2 Diabetes Sprague-Dawley Rat Model. OBES SURG 29, 1889–1900 (2019). https://doi.org/10.1007/s11695-019-03776-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-019-03776-w