Abstract
Background
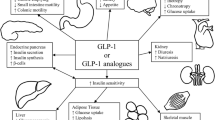
Roux-en-Y gastric bypass (RYGB) is an effective treatment for diabetes. Glucagon-like peptide-1 (GLP-1) is a gut hormone that is important to glucose homeostasis.
Objective
This study aimed to assess GLP-1 level and its predictors after RYGB.
Methods
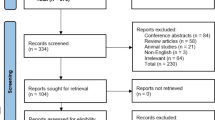
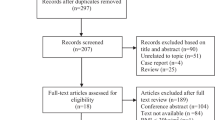
The study design was a meta-analysis. The data sources were MEDLINE, EMBASE, Web of Science, and the Cochrane Databases. The study selection composed of studies with pre- and post-RYGB levels. The main outcomes were as follows: Primary outcome was the change in postprandial GLP-1 levels after RYGB. Secondary outcomes included the changes in fasting glucose, fasting insulin, and fasting GLP-1 levels after RYGB. Meta-regression to determine predictors of changes in GLP-1 levels was performed. Outcomes were reported using Hedge’s g.
Results
Twenty-four studies with 368 patients were included. Postprandial GLP-1 levels increased after RYGB (Hedge’s g = 1.29, p < 0.0001), while fasting GLP-1 did not change (p = 0.23). Peak postprandial GLP-1 levels gave the most consistent results (I 2 = 9.11). Fasting glucose and insulin levels decreased after RYGB (p < 0.0001).
Roux limb length was a significant predictor for amount of GLP-1 increase (β = − 0.01, p = 0.02). Diabetes status, amount of weight loss, length of biliopancreatic limb, and time of measurement were not significant predictors (p > 0.05).
Conclusion
Postprandial GLP-1 levels increase after RYGB, while fasting levels remain unchanged. Shorter Roux limb length is associated with greater increase in postprandial GLP-1, which may lead to better glycemic control in this population.





Similar content being viewed by others
Abbreviations
- RYGB:
-
Roux-En-Y Gastric Bypass
- GLP-1:
-
Glucagon-Like Peptide-1
- BMI:
-
Body Mass Index
- NAFLD:
-
Non-alcoholic Fatty Liver Disease
- US:
-
United States
- SOS:
-
Swedish Obese Subjects
- T2DM:
-
Type 2 Diabetes Mellitus
- STAMPEDE:
-
Surgical Therapy and Medications Potentially Eradicate Diabetes Efficiently
- AUC:
-
Area Under The Curve
- OGTT:
-
Oral Glucose Tolerance Test
- ROBINS-I:
-
Risk Of Bias In Non-Randomized Studies—Of Interventions
- NOS:
-
Newcastle-Ottawa Quality Assessment Scale
- CI:
-
Confidence Interval
- RCT:
-
Randomized Controlled Trial
- FXR:
-
Farnesoid X Receptor
References
WHO | Obesity and overweight. WHO n.d. http://www.who.int/mediacentre/factsheets/fs311/en/ Accessed 16 Aug 2016.
Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011-2014. NCHS Data Brief. 2015:1–8.
Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. J Health Econ. 2012;31:219–30. https://doi.org/10.1016/j.jhealeco.2011.10.003.
Mason EE, Ito C. Gastric bypass in obesity. Surg Clin North Am. 1967;47:1345–51.
Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52. https://doi.org/10.1056/NEJMoa066254.
Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med. 2014;370:2002–13. https://doi.org/10.1056/NEJMoa1401329.
Mason EE. The mechanisms of surgical treatment of type 2 diabetes. Obes Surg. 2005;15:459–61. https://doi.org/10.1381/0960892053723330.
Meek CL, Lewis HB, Reimann F, et al. The effect of bariatric surgery on gastrointestinal and pancreatic peptide hormones. Peptides. 2016;77:28–37. https://doi.org/10.1016/j.peptides.2015.08.013.
Anderwald C-H, Tura A, Promintzer-Schifferl M, et al. Alterations in gastrointestinal, endocrine, and metabolic processes after bariatric Roux-en-Y gastric bypass surgery. Diabetes Care. 2012;35:2580–7. https://doi.org/10.2337/dc12-0197.
Borg C, le Roux C, Ghatei M, et al. Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. Br J Surg. 2006;93:210–5. https://doi.org/10.1002/bjs.5227.
Bose M, Teixeira J, Olivan B, et al. Weight loss and incretin responsiveness improve glucose control independently after gastric bypass surgery. J Diabetes. 2010;2:47–55. https://doi.org/10.1111/j.1753-0407.2009.00064.x.
Breitman I, Saraf N, Kakade M, et al. The effects of an amino acid supplement on glucose homeostasis, inflammatory markers, and incretins after laparoscopic gastric bypass. J Am Coll Surg. 2011;212:617–25. https://doi.org/10.1016/j.jamcollsurg.2010.12.040.
Bryant EJ, King NA, Falken Y, et al. Relationships among tonic and episodic aspects of motivation to eat, gut peptides, and weight before and after bariatric surgery. Surg Obes Relat Dis. 2013;9:802–8. https://doi.org/10.1016/j.soard.2012.09.011.
Campos GM, Rabl C, Peeva S, et al. Improvement in peripheral glucose uptake after gastric bypass surgery is observed only after substantial weight loss has occurred and correlates with the magnitude of weight lost. J Gastrointest Surg. 2010;14:15–22. https://doi.org/10.1007/s11605-009-1060-y.
Chronaiou A, Tsoli M, Kehagias I, et al. Lower ghrelin levels and exaggerated postprandial peptide-YY, glucagon-like peptide-1, and insulin responses, after gastric fundus resection, in patients undergoing Roux-en-Y gastric bypass: a randomized clinical trial. Obes Surg. 2012;22:1761–70. https://doi.org/10.1007/s11695-012-0738-5.
Evans S, Pamuklar Z, Rosko J, et al. Gastric bypass surgery restores meal stimulation of the anorexigenic gut hormones glucagon-like peptide-1 and peptide YY independently of caloric restriction. Surg Endosc Interv Tech. 2012;26:1086–94. https://doi.org/10.1007/s00464-011-2004-7.
Falken Y, Hellstrom PM, Holst JJ, et al. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J Clin Endocrinol Metab. 2011;96:2227–35. https://doi.org/10.1210/jc.2010-2876.
Fellici AC, Lambert G, Lima MMO, et al. Surgical treatment of type 2 diabetes in subjects with mild obesity: mechanisms underlying metabolic improvements. Obes Surg. 2015;25:36–44. https://doi.org/10.1007/s11695-014-1377-9.
Gandolfini M-P, Coupaye M, Bouaziz E, et al. Cardiovascular changes after gastric bypass surgery: involvement of increased secretions of glucagon-like peptide-1 and brain natriuretic peptide. Obes Surg. 2015;25:1933–9. https://doi.org/10.1007/s11695-015-1643-5.
Hansen EN, Tamboli RA, Isbell JM, et al. Role of the foregut in the early improvement in glucose tolerance and insulin sensitivity following Roux-en-Y gastric bypass surgery. Am J Physiol-Gastrointest LIVER Physiol. 2011;300:G795–802. https://doi.org/10.1152/ajpgi.00019.2011.
Jorgensen NB, Jacobsen SH, Dirksen C, et al. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with type 2 diabetes and normal glucose tolerance. Am J Physiol-Endocrinol Metab. 2012;303:E122–31. https://doi.org/10.1152/ajpendo.00073.2012.
Korner J, Inabnet W, Febres G, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes. 2009;33:786–95. https://doi.org/10.1038/ijo.2009.79.
Lips MA, de Groot GH, van Klinken JB, et al. Calorie restriction is a major determinant of the short-term metabolic effects of gastric bypass surgery in obese type 2 diabetic patients. Clin Endocrinol. 2014;80:834–42. https://doi.org/10.1111/cen.12254.
Morinigo R, Lacy AM, Casamitjana R, et al. GLP-1 and changes in glucose tolerance following gastric bypass surgery in morbidly obese subjects. Obes Surg. 2006;16:1594–601. https://doi.org/10.1381/096089206779319338.
Nosso G, Griffo E, Cotugno M, et al. Comparative effects of Roux-en-Y gastric bypass and sleeve gastrectomy on glucose homeostasis and incretin hormones in obese type 2 diabetic patients: a one-year prospective study. Horm Metab Res. 2016;48:312–7. https://doi.org/10.1055/s-0041-111505.
O’Brien CS, Wang G, McGinty J, et al. Effects of gastrogastric fistula repair on weight loss and gut hormone levels. Obes Surg. 2013;23:1294–301. https://doi.org/10.1007/s11695-013-0917-z.
Peterli R, Steinert RE, Woelnerhanssen B, et al. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg. 2012;22:740–8. https://doi.org/10.1007/s11695-012-0622-3.
Promintzer-Schifferl M, Prager G, Anderwald C, et al. Effects of gastric bypass surgery on insulin resistance and insulin secretion in nondiabetic obese patients. Obesity. 2011;19:1420–6. https://doi.org/10.1038/oby.2011.92.
Rubino F, Gagner M, Gentileschi P, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240:236–42. https://doi.org/10.1097/01.sla.0000133117.12646.48.
Salinari S, Bertuzzi A, Guidone C, et al. Insulin sensitivity and secretion changes after gastric bypass in normotolerant and diabetic obese subjects. Ann Surg. 2013;257:462–8. https://doi.org/10.1097/SLA.0b013e318269cf5c.
Samat A, Malin SK, Huang H, et al. Ghrelin suppression is associated with weight loss and insulin action following gastric bypass surgery at 12 months in obese adults with type 2 diabetes. Diabetes Obes Metab. 2013;15:963–6. https://doi.org/10.1111/dom.12118.
Steven S, Hollingsworth KG, Small PK, et al. Weight loss decreases excess pancreatic triacylglycerol specifically in type 2 diabetes. Diabetes Care. 2016;39:158–65. https://doi.org/10.2337/dc15-0750.
Umeda LM, Silva EA, Carneiro G, et al. Early improvement in glycemic control after bariatric surgery and its relationships with insulin, GLP-1, and glucagon secretion in type 2 diabetic patients. Obes Surg. 2011;21:896–901. https://doi.org/10.1007/s11695-011-0412-3.
Yan W, Polidori D, Yieh L, et al. Effects of meal size on the release of GLP-1 and PYY after Roux-en-Y gastric bypass surgery in obese subjects with or without type 2 diabetes. Obes Surg. 2014;24:1969–74. https://doi.org/10.1007/s11695-014-1316-9.
Yip S, Signal M, Smith G, et al. Lower glycemic fluctuations early after bariatric surgery partially explained by caloric restriction. Obes Surg. 2014;24:62–70. https://doi.org/10.1007/s11695-013-1043-7.
Yousseif A, Emmanuel J, Karra E, et al. Differential effects of laparoscopic sleeve gastrectomy and laparoscopic gastric bypass on appetite, circulating acyl-ghrelin, peptide YY3-36 and active GLP-1 levels in non-diabetic humans. Obes Surg. 2014;24:241–52. https://doi.org/10.1007/s11695-013-1066-0.
Zhang X, Cheng Z, Xiao Z, et al. Comparison of short- and mid-term efficacy and the mechanisms of gastric bypass surgeries on managing obese and nonobese type 2 diabetes mellitus: a prospective study. Arch Med Res. 2015;46:303–9. https://doi.org/10.1016/j.arcmed.2015.06.003.
Laferrere B. Effect of gastric bypass surgery on the incretins. Diabetes Metab. 2009;35:513–7.
Nausheen S, Shah IH, Pezeshki A, et al. Effects of sleeve gastrectomy and ileal transposition, alone and in combination, on food intake, body weight, gut hormones, and glucose metabolism in rats. Am J Physiol Endocrinol Metab. 2013;305:E507–18. https://doi.org/10.1152/ajpendo.00130.2013.
Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244:741–9. https://doi.org/10.1097/01.sla.0000224726.61448.1b.
Penney NC, Kinross J, Newton RC, et al. The role of bile acids in reducing the metabolic complications of obesity after bariatric surgery: a systematic review. Int J Obes. 2015;39:1565–74. https://doi.org/10.1038/ijo.2015.115.
Kohli R, Bradley D, Setchell KD, et al. Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J Clin Endocrinol Metab. 2013;98:E708–12. https://doi.org/10.1210/jc.2012-3736.
Ahmad NN, Pfalzer A, Kaplan LM. Roux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. Int J Obes. 2013;37:1553–9. https://doi.org/10.1038/ijo.2013.38.
Gerhard GS, Styer AM, Wood GC, et al. A role for fibroblast growth factor 19 and bile acids in diabetes remission after Roux-en-Y gastric bypass. Diabetes Care. 2013;36:1859–64. https://doi.org/10.2337/dc12-2255.
Gleysteen JJ. Five-year outcome with gastric bypass: Roux limb length makes a difference. Surg Obes Relat Dis Off J Am Soc Bariatr Surg. 2009;5:242–247; discussion 247-249. https://doi.org/10.1016/j.soard.2008.08.005.
Stefanidis D, Kuwada TS, Gersin KS. The importance of the length of the limbs for gastric bypass patients—an evidence-based review. Obes Surg. 2011;21:119–24. https://doi.org/10.1007/s11695-010-0239-3.
Orci L, Chilcott M, Huber O. Short versus long Roux-limb length in Roux-en-Y gastric bypass surgery for the treatment of morbid and super obesity: a systematic review of the literature. Obes Surg. 2011;21:797–804. https://doi.org/10.1007/s11695-011-0409-y.
Dogan K, Homan J, Aarts EO, et al. A short or a long Roux limb in gastric bypass surgery: does it matter? Surg Endosc. 2016; https://doi.org/10.1007/s00464-016-5188-z.
Kaska L, Kobiela J, Proczko M, et al. Does the length of the biliary limb influence medium-term laboratory remission of type 2 diabetes mellitus after Roux-en-Y gastric bypass in morbidly obese patients? Wideochirurgia Inne Tech Małoinwazyjne Videosurgery Miniinvasive Tech Kwart Pod Patronatem Sekc Wideochirurgii TChP Oraz Sekc Chir Bariatrycznej TChP. 2014;9:31–9. https://doi.org/10.5114/wiitm.2014.40383.
Gupta RVN, Chamany T, Makam R. Does length of common limb influence remission of diabetes? Short-term results. J Minimal Access Surg. 2016;12:54–7. https://doi.org/10.4103/0972-9941.152104.
Dutra RA, Araújo WM, de Andrade JI. The effects of Roux-en-Y limb length on gastric emptying and enterogastric reflux in rats. Acta Cirúrgica Bras Soc Bras Para Desenvolv Pesqui Em Cir. 2008;23:179–83.
Le Blanc-Louvry I, Ducrotté P, Lemeland JF, et al. Motility in the Roux-Y limb after distal gastrectomy: relation to the length of the limb and the afferent duodenojejunal segment—an experimental study. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc. 1999;11:365–74.
Funding
No financial or material support was received for this research project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval Statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent Statement
Does not apply.
Rights and permissions
About this article
Cite this article
Jirapinyo, P., Jin, D.X., Qazi, T. et al. A Meta-Analysis of GLP-1 After Roux-En-Y Gastric Bypass: Impact of Surgical Technique and Measurement Strategy. OBES SURG 28, 615–626 (2018). https://doi.org/10.1007/s11695-017-2913-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-017-2913-1