Abstract
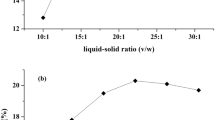
The optimization of extraction of pectin from unripe grape pomace (PUGP) was studied using response surface methodology (RSM) and Box Behnken design (BBD). Conventional and ultrasound-assisted extractions (CE and UAE) were considered to optimize the pH, temperature and time conditions. For CE method, pH, temperature and time were 1–3, 60–90 °C, and 60–120 min while for UAE, 1–3, 50–70 °C, and 10–30 min, respectively. Extraction yield (EY, %), degree of esterification (ES, %), and galacturonic acid (GA, %) were optimized. The optimum conditions for CE were 2.95, 80.27 °C, and 120 min that led to 18.48% of EY, 43.49% of DE, and 53.76% of GA while for UAE were 3, 58.84 °C, and 30 min that led to 28.43%, 31.02%, and 63.94%, respectively. Emulsifying activity (EA, %) and emulsifying stability (ES, %) of optimum PUGP of UAE method were higher (36.10% and 70.71%) than those obtained for PUGP of CE (25.59% and 55.17%), while both were lower than those of commercial apple (63.23% and 88.67%) and citrus pectins (72.13% and 92.94%). Fourier transform infrared spectroscopy analysis of PUGP of UAE confirmed lower DE than PUGP of CE. Scanning electron microscopy of PUGP of UAE indicated disintegrated regions attributed to sonication effects. Differential scanning calorimetry exhibited that the thermal stability of PUGP of UAE was lower than that obtained for PUGP of CE. Overall, UAE led to extraction of pectin from PUGP at a significant low time, high EY and GA, and low DE, which can be incorporated into dairy products.






Similar content being viewed by others
References
M. Spinei, M. Oroian, Int. J. Biol. Macromol. 224, 739 (2023)
A.S. Sengar, A. Rawson, M. Muthiah, S.K. Kalakandan, Ultrason. Sonochem. 61, 104812 (2020)
S. Paidari, N. Zamindar, R. Tahergorabi, M. Kargar, S. Ezzati, N. shirani, S.H. Musavi, J. Food Meas. Charact. 15, 4205 (2021)
F. Xu, S. Zhang, G.I.N. Waterhouse, T. Zhou, Y. Du, D. Sun-Waterhouse, P. Wu, Food Hydrocoll. 133, 107945 (2022)
R. Minjares-Fuentes, A. Femenia, M.C. Garau, J.A. Meza-Velázquez, S. Simal, C. Rosselló, Carbohydr. Polym. 106, 179 (2014)
T.A. Thu Dao, H.K. Webb, F. Malherbe, Food Hydrocoll. 113, 106475 (2021)
A. Kashani, M. Hasani, L. Nateghi, M.J. Asadollahzadeh, P. Kashani, Iran. J Chem Chem Eng. 41, 1288 (2022)
L. Nateghi, F. Zarei, M. Zarei, Iran. J Chem Chem Eng (2022)
H. Mirzaee, F. Khodaiyan, J.F. Kennedy, S.S. Hosseini, Carbohydr. Polym. Technol. Appl. 1, 100004 (2020)
S. paidari, H. Ahari, A. Pasqualone, A.A. Anvar, S. Allah Yari Beyk, and, S. Moradi, J. Food Meas. Charact. 17, 2595 (2023)
Y. Esmaeili, S. Paidari, S.A. Baghbaderani, L. Nateghi, A.A. Al-Hassan, F. Ariffin, J. Food Meas. Charact. 16, 507 (2022)
E.E. Santos, R.C. Amaro, C.C.C. Bustamante, M.H.A. Guerra, L.C. Soares, R.E.S. Froes, Food Hydrocoll. 107, 105921 (2020)
S. Ezzati, A. Ayaseh, B. Ghanbarzadeh, M.K. Heshmati, Int. J. Biol. Macromol. 165, 776 (2020)
C. Colodel, L.C. Vriesmann, R.F. Teófilo, C.L. de Oliveira, Petkowicz, Int. J. Biol. Macromol. 161, 204 (2020)
T.M. Pellicanò, A.M. Giuffrè, C. Zappia, M. Capocasale, (n.d.).
J. Yu, M. Ahmedna, Int. J. Food Sci. Technol. 48, 221 (2013)
S.R.F. Iora, G.M. Maciel, A.A.F. Zielinski, M.V. da Silva, P.V. Pontes, C.W.I. Haminiuk, D. Granato, Int. J. Food Sci. Technol. 50, 62 (2015)
G. Ruberto, A. Renda, C. Daquino, V. Amico, C. Spatafora, C. Tringali, N. De Tommasi, Food Chem. 100, 203 (2007)
M. Spinei, M. Oroian, Foods 2021, Vol. 10, Page 867 10, 867 (2021)
P. Van Hung, M.N.T. Anh, P.N. Hoa, N.T.L. Phi, J. Food Meas. Charact. 15, 1541 (2021)
M.M. Alancay, M.O. Lobo, C.M. Quinzio, L.B. Iturriaga, J. Food Meas. Charact. 11, 2119 (2017)
D.L. Su, P.J. Li, S.Y. Quek, Z.Q. Huang, Y.J. Yuan, G.Y. Li, Y. Shan, Food Chem. 286, 1 (2019)
A. Rezvankhah, Z. Emam-Djomeh, M. Safari, G. Askari, M. Salami, J. Food Sci. Technol. 56, 4198 (2019)
S.A. Mousavi, L. Nateghi, M. Javanmard Dakheli, Y. Ramezan, Z. Piravi-Vanak, S. Paidari, A. Mohammadi, Nafchi, J. Food Meas. Charact. 16, 4236 (2022)
A. Rezvankhah, Z. Emam-Djomeh, M. Safari, G. Askari, M. Salami, J. Food Process. Preserv 42, (2018)
K. Asgari, M. Labbafi, F. Khodaiyan, M. Kazemi, S.S. Hosseini, Int. J. Biol. Macromol. 152, 1274 (2020)
D.A. Mendez, M.J. Fabra, A. Martínez-Abad, M. Μartínez-Sanz, Gorria, and A. López-Rubio, Food Hydrocoll. 120, 106957 (2021)
15, 91 (2019)
I.G. Moorthy, J.P. Maran, S. Ilakya, S.L. Anitha, S.P. Sabarima, B. Priya, Ultrason. Sonochem. 34, 525 (2017)
W. Wang, X. Ma, P. Jiang, L. Hu, Z. Zhi, J. Chen, T. Ding, X. Ye, D. Liu, Food Hydrocoll. 61, 730 (2016)
A. Rezvankhah, M.S. Yarmand, B. Ghanbarzadeh, H. Mirzaee, J. Food Process. Preserv. 45, e15932 (2021)
C.S. Shivamathi, I.G. Moorthy, R.V. Kumar, M.R. Soosai, J.P. Maran, R.S. Kumar, P. Varalakshmi, Carbohydr. Polym. 225, 115240 (2019)
I.G. Moorthy, J.P. Maran, S.M. Surya, S. Naganyashree, C.S. Shivamathi, Int. J. Biol. Macromol. 72, 1323 (2015)
W.W. Wai, A.F.M. Alkarkhi, A.M. Easa, Food Bioprod. Process. 88, 209 (2010)
S.H. Jong, N. Abdullah, N. Muhammad, Carbohydr. Polym. Technol. Appl. 5, 100263 (2023)
T.Ã.S. Oliveira, M.F. Rosa, F.L. Cavalcante, P.H.F. Pereira, G.K. Moates, N. Wellner, S.E. Mazzetto, K.W. Waldron, H.M.C. Azeredo, Food Chem. 198, 113 (2016)
M. Masmoudi, S. Besbes, M. Chaabouni, C. Robert, M. Paquot, C. Blecker, H. Attia, Carbohydr. Polym. 74, 185 (2008)
J. Chen, H. Cheng, Z. Zhi, H. Zhang, R.J. Linhardt, F. Zhang, S. Chen, X. Ye, Food Hydrocoll. 112, 106160 (2021)
K. Kumar, S. Srivastav, V.S. Sharanagat, Ultrason. Sonochem. 70, 105325 (2021)
C.F. de Oliveira, D. Giordani, R. Lutckemier, P.D. Gurak, F. Cladera-Olivera, L.D.F. Marczak, LWT-Food Sci. Technol. 71, 110 (2016)
E. Polanco-Lugo, J.I. Martínez-Castillo, J.C. Cuevas-Bernardino, T. González-Flores, R. Valdez-Ojeda, N. Pacheco, T. Ayora-Talavera, Http://Mc.Manuscriptcentral.Com/Tcyt. 17, 463 (2019)
B.M.N. Nguyen, T. Pirak, Http://Www.Editorialmanager.Com/Cogentagri 5, (2019)
R.M. Zaid, P. Mishra, A.R. Siti Noredyani, S. Tabassum, Z. Ab, Wahid, A.M. Mimi, Sakinah, Food Bioprod. Process. 123, 134 (2020)
A. Rezvankhah, Z. Emam-Djomeh, M. Safari, M. Salami, G. Askari, J. Food Process. Preserv e16554 (2022)
E. Kliemann, K.N. De Simas, E.R. Amante, E.S. Prudêncio, R.F. Teófilo, M.M.C. Ferreira, R.D.M.C. Amboni, Int. J. Food Sci. Technol. 44, 476 (2009)
M. Spinei, M. Oroian, Sci Rep. 2022. 121(12), 1 (2022)
M. Spinei, M. Oroian, Polym. 2022, Vol. 14, Page 1378 14, 1378 (2022)
J.P. Maran, B. Priya, Carbohydr. Polym. 115, 732 (2015)
Y.S. Duwee, P.L. Kiew, W.M. Yeoh, J. Food Meas. Charact. 16, 1710 (2022)
A. Rezvankhah, M.S. Yarmand, B. Ghanbarzadeh, H. Mirzaee, J. Food Meas. Charact. 15, 5021 (2021)
A. Rezvankhah, S. Mohammad, Yarmand, | Babak Ghanbarzadeh, and | Homaira Mirzaee, Food Sci. Nutr. 00, 1 (2023)
E.D. Ngouémazong, S. Christiaens, A. Shpigelman, A. Van Loey, M. Hendrickx, Compr. Rev. Food Sci. Food Saf. 14, 705 (2015)
A. Rezvankhah, M.S. Yarmand, B. Ghanbarzadeh, J. Food Meas. Charact. 1 (2022)
M. Marhamati, G. Ranjbar, M. Rezaie, J. Mol. Liq. 340, 117218 (2021)
L. Wan, Q. Chen, M. Huang, F. Liu, S. Pan, Food Hydrocoll. 93, 146 (2019)
X. Yang, T. Nisar, Y. Hou, X. Gou, L. Sun, Y. Guo, Food Hydrocoll. 85, 30 (2018)
S.H.E. Verkempinck, C. Kyomugasho, L. Salvia-Trujillo, S. Denis, M. Bourgeois, A.M. Van Loey, M.E. Hendrickx, T. Grauwet, Food Hydrocoll. 85, 144 (2018)
A. Rezvankhah, Z. Emam-Djomeh, G. Askari, Dry. Technol. 38, 235 (2020)
Z. Emam-Djomeh, A. Rezvankhah, Release Bioavailab. Nanoencapsulated Food Ingredients (Elsevier, 2020), pp. 79–120
Z. Rahmani, F. Khodaiyan, M. Kazemi, A. Sharifan, Int. J. Biol. Macromol. 147, 1107 (2020)
W. Wang, X. Ma, Y. Xu, Y. Cao, Z. Jiang, T. Ding, X. Ye, D. Liu, Food Chem. 178, 106 (2015)
N. Haghighatpanah, H. Mirzaee, F. Khodaiyan, J.F. Kennedy, A. Aghakhani, S.S. Hosseini, K. Jahanbin, Int. J. Biol. Macromol. 152, 305 (2020)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of authors declare conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
vakilian, K., Nateghi, L., Javadi, A. et al. Optimization of conventional and ultrasound-assisted extraction of pectin from unripe grape pomace: extraction yield, degree of esterification, and galacturonic acid content. Food Measure 17, 5777–5793 (2023). https://doi.org/10.1007/s11694-023-02085-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-023-02085-2