Abstract
Juices produced on an industrial scale are largely devoid of pectin substances, and lack a significant part of their vitamins, carotenoids and polyphenols. In recent years, physical methods have been used for preliminary treatments prior to pressing, allowing the elimination of enzymatic treatment. This paper presents the results of a study on the effect of freezing and thawing, as a preliminary treatment, on the efficiency of pressing and on the quality of juice obtained from northern highbush blueberry. Preliminary treatment of pulp, consisting in its freezing and thawing, caused a notable (23.1%), but lower than that for enzymatic treatment (34.9%), increase in pressing efficiency relative to control samples. The highest content of lutein and vitamin C was obtained in juice acquired from pulp subjected to treatment consisting in its freezing and thawing prior to pressing. The application of enzymatic treatment caused a 26.9% increase in the content of juice extract, while the treatment consisting in freezing and thawing caused a 19.5% increase in the content of extract. Irrespective of the type of preliminary treatment applied, a degradation of l-ascorbic acid and an increase in the content of dehydro-l-ascorbic acid were observed in the juices produced. The method proposed permits the production of juices with health-promoting properties, due to the application of freezing as a preliminary treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Compounds contained in fruits and vegetables which have documented health-promoting effects include carotenoids, flavonoids, and vitamins. One of the sources of lutein and vitamin C is northern highbush blueberry (Vaccinium corymbosum L.). Lutein and vitamin C have a considerable nutritional value due to their antioxidant and anti-carcinogenic effects, and because they protect the organism against the cardio-vascular and nervous systems disorders [1]. Lutein and zeaxanthin, by limiting oxidative stress, protect sensitive eye tissue against disorders related with age-related macular degeneration (AMD) and retinitis pigmentosa (RP), both of which are light-induced, and against cataracts [2, 3]. Lutein and zeaxanthin are not produced in the human organism and must be supplied from outside, preferably with food. The content of lutein and vitamin C in fruits and vegetables varies. According to Marinova and Ribarova [4], the content of lutein is 0.21 mg/100 g in black currant, 0.27 mg/100 g in blackberry and 0.32 mg/100 g in raspberry. The highest content of lutein among berries, amounting to 1.53 mg/100 g, has been assayed in blueberry [5].
The name vitamin C can refer to both l-ascorbic acid (AA) and dehydro-l-ascorbic acid (DHAA). DHAA displays the properties of vitamin C, as it is easily reduced in the organism to AA [6]. The content of vitamin C in fruits and vegetables varies strongly. The content of vitamin C in plants is affected by numerous factors: genetic variability, ripeness, climate, methods of cultivation, harvest and storage [7]. In general, the processes of fruit and vegetable processing and the conditions of storage of raw materials and products cause a gradual decrease in the content of this vitamin [8].
Blueberries in fresh form consist of water (84%), carbohydrates (9.7%), proteins (0.6%) and fat (0.4%) [9]. The average content of vitamin C in blueberries is 8.6 mg/100 g [8]. The total content of polyphenols in blueberries ranges from 48 up to 304 mg/100 g of fresh fruit weight [10]. As can be seen in the literature, the composition and content of polyphenols occurring in northern highbush blueberry is a relatively well researched topic [11, 12], while there are few publications on the content of carotenoids, and no information on the content of lutein in the fruits of the blueberry.
Northern highbush blueberry is consumed in unprocessed form as fresh fruit, and also in processed forms such as frozen or dried products, jams, jellies, juices, canned fruit, and puree [9, 13]. Blueberry processing causes a significant decrease in the content of health-promoting components in the fruits [14, 15]. The literature provides information on the behavior of flavonoids during the processing of northern highbush blueberry fruits [13, 16, 17]. However, there is no information on how processing affects carotenoids, including lutein, contained in blueberry.
One of the more often used forms of northern highbush blueberry processing is the production of juices with the method of pressure extraction [16, 18]. With regard to the degree of preservation of substances valuable for human health, the number and kind of unit processing operations performed in the production process are of importance [19]. The typical technological processes used in fruit juice production on an industrial scale include washing, inspection, mashing, enzymatic liquefaction, pressing, thermal treatment, depectinization, clarification, filtration, pasteurization and packing [20]. Juices produced industrially are largely devoid of pectin substances, a notable part of vitamins, carotenoids and polyphenols. Commercial enzymes cause a certain degree of deglycosylation of anthocyanins and other polyphenols and this leads to their destabilization [21, 22]. In recent years, physical methods have been used for preliminary treatments prior to pressing, allowing the elimination of enzymatic treatment. Preliminary treatment consisting in pulp freezing and thawing before pressing causes an improvement of the process efficiency and permits the preservation of health-promoting components, and does not cause any deterioration of the sensory traits of the juices [23, 24].
The aim of this study was to determine the influence of initial processing consisting in the use of freezing and thawing techniques on the efficiency and quality of juice yielded by pressing using laboratory press. The scope of the study included the determination both of the efficiency of pressing, depending on the pre-treatment type, and of the quantitative parameters of the yielded juice, such as the content of extract, its density, and acidity, and the levels of organic acid, lutein and vitamin C.
Materials and methods
Sample preparations
The study was conducted using the northern highbush blueberry variety Nelson, harvested in 2014 and supplied by a specialist horticulture farm in Matcze (50°56′52″N, 23°58′6″E). The fruits were harvested at the end of September. The initial moisture content of blueberry fruits was 85.1%. The material accepted for the analyses was healthy, without any mechanical damage. The blueberry fruits were washed, dried by blotting, and then mashed with the use of a twin-cylinder crusher. The resultant pulp was divided into 300-g portions which were packed in plastic containers. Plastic containers with pulp were divided into three parts. The first group of plastic containers was the control sample without any pre-treatment. The second was frozen and kept at − 20 ± 1 °C in a standard air freezer (F6243W, Gorenje Group, Velenje, Slovenia) for 24 h and, before pressing, was thawed at 20 ± 1 °C using an incubator (SUP-4, Wamed, Warsaw, Poland). A standard thawing time of 6 h ensured complete thawing of the mash. The third part of the pulp after adding an enzymatic preparation (Pektoenzym, Biowin sp. z o.o., Łódź, Poland) in the amount of 0.2 ml per 1 kg, was placed (in plastic containers) in an incubator at 25 ± 1 °C for a period of 4 h.
Juice pressing process
The northern highbush blueberry was pressed in a laboratory hydraulic basket press with a working chamber volume of approximately 1 dcm3 according to Nadulski et al. [24]. The press consisted of a base, a perforated cylinder with holes of 3 mm diameter, a piston, a frame structure, a hydraulic ram (UHJG 20/C/2, Hydrotech, Lublin, Poland) and a measurement system consisting of a tensometer (EMS50, WObit, Poznań, Poland), operating together with a digital recorder (MG-TAE1, WObit, Poznań, Poland). A 300-g sample of blueberry pulp was placed into a filter bag and then in the press cylinder, and then pressed using the piston. The speed of piston displacement was 0.5 mm s−1. After reaching a force of 45 kN, the pressing was stopped. Each pressing was performed 10 times.
Treatment procedures
The material for pressing was prepared according to the procedures shown in Fig. 1. The first procedure (P1) consisted in the pressing of pulp obtained from blueberry fruits. The second procedure (P2) consisted in the pressing of blueberry fruit pulp after its prior freezing and thawing. In the third procedure (P3), blueberry pulp subjected to enzymatic treatment was pressed. Pomace obtained in P1 and P2 was slowly frozen at a temperature of − 20 ± 1 °C and kept frozen for 24 h, then thawed over a period of 6 h at a temperature of 20 ± 1 °C and subjected to pressing (procedures P1W and P2W). Analyses were conducted for fresh juice, without any thermal treatment. Measurements were taken at an ambient temperature of 22 ± 10 °C. Ten samples were used for each experimental condition.
Schematic diagram of the experiment. Procedure P1—one-time pressing of highbush blueberry pulp. Procedure P2—one-time pressing of highbush blueberry pulp after freezing and thawing. Procedure P3—one-time pressing of highbush blueberry pulp after enzymatic treatment. Procedure P1W—one-time pressing of highbush blueberry pomace (obtained with procedure P1) after freezing and thawing. Procedure P2W—one-time pressing of highbush blueberry pomace (obtained with procedure P2) after freezing and thawing. C—juice from fruit pulp subjected to one-time pressing (procedure P1). F—juice from fruit pulp after freezing and thawing, subjected to one-time pressing (procedure P2). E—juice from fruit pulp after enzymatic liquefaction, subjected to one-time pressing (procedure P3). CP—juice from pomace subjected to freezing and thawing prior to second pressing (procedure P1W). FP—juice from pomace obtained from frozen and thawed pulp, subjected to freezing and thawing prior to second pressing (P2W)
Determination of pressing efficiency
The efficiency of pressing the blueberry mash was calculated using the formula:
where W is the efficiency of pressing blueberry mash, %; Mj is the mass of juice after pressing, kg; and Mi is the mass of input material, kg.
Determination of extract content
After each extraction, soluble solids content (PN-EN 12143:2000) [25] was established. To determine the sugar content (°Bx) of the extracted juice, a PAL-1 refractometer (Atago, Tokyo, Japan) was used. Each measurement was conducted in five replications.
Determination of the titratable acidity
The acidity of the obtained juice was determined analytically using a method for the determination of titratable acidity in fruit and vegetable juices using potentiometric titration of the product with a standardized sodium hydroxide water solution. Each measurement was conducted in five replications. Titratable acidity was converted to tartaric acid, malic acid and citric acid according PN-EN 12147:2000 [26].
Determination of the content of vitamin C
The content of vitamin C was assayed in accordance with the method proposed by Mazurek and Jamroz [27]. A reverse-phase HPLC method was used for the determination of the total content of vitamins C and AA. In the first stage, the content of AA was analyzed in the sample and then DHA was reduced quantitatively using tris(2-carboxyethyl)phosphine and the total content of vitamin C was determined. The concentration of DHA was calculated by subtracting the initial concentration of AA from the total concentration of vitamin C (differential method). The analyses were performed with the use of a Varian (USA) HPLC system equipped with a diode-array detector (DAD, type 335), an isocratic pump (type 210), a 7725i dosing valve (Rheodyne, USA) and a column thermostat. Galaxie Chromatography Data System version 1.9.302 (Varian, USA) was used for process control and data collection. Separations were made using a Gemini column (150 × 4.6 mm, 3 μm, C18, Phenomenex, USA) connected with a Gemini pre-column (4 × 3 mm, C18, Phenomenex, USA). The injection volume was 20 μL. The mobile phase was a solution of orthophosphoric acid at pH 2.8, pumped at a flow of 0.6 mL/min. Chromatograms were recorded at 244 nm and a column temperature of 30 °C. AA identification was performed on the basis of the retention time and UV absorption spectrum of the standard sample. The concentration of AA was calculated from the equation of the calibration curve plotted for the standard solutions. Each measurement was conducted in five replications.
Determination of lutein content
Lutein isolation and quantity determination were performed using HPLC (HP, USA). Extracts of carotenoids were obtained from juices (10 mL) that were shaken with a mixture of methyl tert-butyl ether and methanol. As a result, after centrifugation, an ether extract with a mixture of carotenoid pigments was obtained. The extract was concentrated in a vacuum evaporator to the point of total evaporation of liquid, and then dissolved in 1 mL of chromatographic phase and subjected to analysis. Lutein was assayed by using HPLC with an HP 1050 UV detector and an HR 80 column (RP-C18), following the modification of the method given by Caldwell and Britz [28] and Mou [29]. Elution was performed with the use of an acetonitrile solvent (80%), water (15%) and ethyl acetate (5%) with triethylamine (0.05%). Data logging was conducted at a wavelength of λ = 460 nm. Compounds were identified on the basis of retention times compared to standards. Each measurement was conducted in five replications.
Statistical analysis
Statistical analysis of the data was performed with Statistica software [30], using analysis of variance for factorial designs. The significance of differences was tested using Tukey’s LSD test. The results are presented in graphs and tables. The graphs show mean values and whiskers representing standard deviations. The tables present mean values and standard deviations.
Results and discussion
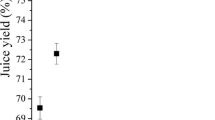
The study demonstrated that the pressing efficiency and the quality of the juice produced depend on the kind of pulp pre-treatment prior to the pressing. Application of the enzymatic treatment or freezing and thawing of pulp prior to pressing caused a statistically significant increase in pressing efficiency (Fig. 2). In the case of enzymatic-liquefied pulp (P3), a 34.9% increase in pressing efficiency was obtained relative to the control sample (P1), while in the case of pulp frozen and thawed prior to pressing (P2) the increase in pressing efficiency was 23.1% in relation to the control sample (P1). The increase in extraction efficiency of pressing after the enzymatic treatment is associated with transformation of the structural ingredients within the pulp. The mechanism of action of the enzyme on fruit pulp to improve the effectiveness of pressure extraction is relatively well understood [31]. The increase in pressing efficiency after freezing and thawing is related to the formation of ice crystals during the freezing of fruit pulp. Slow freezing causes a gradual increase in the size of the ice crystals, as a result of which damage to the tissue structure is observed [32]. That damage causes a loss of cell turgor after thawing, and considerable seepage of cellular juice. During the pressing, the structural damage resulting from freezing intensifies the deformation and cracking of cells and causes a reduction of the resistance of cellular juice flow through the layer of the material being pressed.
Efficiency of pressing Wj in relation to treatment procedure applied. Procedure P1—one-time pressing of highbush blueberry pulp. Procedure P2—one-time pressing of highbush blueberry pulp after freezing and thawing. Procedure P3—one-time pressing of highbush blueberry pulp after enzymatic treatment. a, b, c average values marked with the same letter are not statistically significantly different (p ≤ 0.05). Data represent mean values of 10 replications
Pomace obtained in P1 and P2 after the first pressing was frozen and thawed, and then pressed. The additional pressing of pomace after freezing and thawing caused a 34.3% increase in the total process efficiency in the case of pomace obtained from the first pressing of pulp after freezing and thawing, and a 68.4% increase in the case of pomace originating from the control sample after freezing and thawing (Fig. 3). This efficiency is similar to the values presented by Lee et al. [16] for blueberry. By augmenting the pressing with enzymatic treatment and an addition of rice hulls, the authors achieved a process efficiency of 75–83%.
Total efficiency of pressing Wj in relation to treatment procedure applied, with the inclusion of processing of pomace obtained after the first pressing. Procedure P1—one-time pressing of highbush blueberry pulp. Procedure P2—one-time pressing of highbush blueberry pulp after freezing and thawing. Procedure P1W—one-time pressing of highbush blueberry pomace (obtained with procedure P1) after freezing and thawing. Procedure P2W—one-time pressing of highbush blueberry pomace (obtained with procedure P2) after freezing and thawing. a, b average values marked with the same letter are not statistically significantly different (p ≤ 0.05). Data represent mean values of 10 replications
Our study demonstrated that the content of extract in juice from northern highbush blueberry depends on the method of juice extraction (Table 1). The lowest value of extract content, 8.43oBx, was recorded in the case of juice pressed from fruits mashed by means of the crusher (P1). According to Sinelli et al. [33], the average extract content in juice obtained from squeezing berries is 8.42oBx. The content of extract in blueberry fruits can be higher and depends on the stage of ripeness and on the variety traits of the fruits. High values of extract content, above 14oBx, have been obtained in juice acquired from frozen highbush blueberry with the use of enzymatic treatment [16]. The use of preliminary treatment consisting of pulp freezing and thawing in the course of the experiment resulted in an average 19.5% increase in extract content in the juice. The use of frozen fruits in the production of blueberry juice increases the degree of extraction of sugars, anthocyanin pigments and other components [34].
The study showed a distinct effect of preliminary treatment on the content of lutein in highbush blueberry juice (Table 2). In the case of juice from fruits subjected to crushing alone (P1), a small amount of lutein was detected. The main source of lutein in blueberry fruits can be the fruit skin. The mashing of highbush blueberry fruits with a twin-cylinder crusher does not cause the rupture of fruit skin cells and lutein migration to the juice. Carotenoids contained in fruits and vegetables accumulate in the skin and outer leaves. As an example, the outer leaves of white cabbage contain ca. 150-fold more lutein than the inner leaves [35]. During the study, the highest content of lutein was obtained for freezing and thawing as pulp pre-treatment prior to pressing (P2). This is related to cell cracking in the course of the freezing and thawing, which facilitates the transition of components to the cellular juice. Bunea et al. [36] indicate that the main carotenoids identified in blueberries are lutein, β-cryptoxanthin, and β-carotene, with an average total carotenoid content of 0.266 mg per 100 g of fruit. Using procedure P2 a lutein content of 0.28 mg/100 g was obtained. Enzymatic treatment (P3) causes the liquefaction of pulp, but no significant damage to fruit skin and, as a result, has a lesser effect on increasing the level of lutein in juice compared to P2. The application of procedures P1W and P2W permits the acquisition of lutein from the pomace (Table 2).
In the fresh fruits taken for the tests, the total content of vitamin C was 6.75 ± 1.06 mg/100 g, including AA—5.41 ± 0.96 mg/100 g and DHAA—1.34 ± 0.34 mg/100 g. The treatment applied had a significant effect on the content of vitamin C in the juice (Table 2). Irrespective of the kind of preliminary treatment (P1, P2 or P3), there was a decrease in the content of vitamin C. The highest content of vitamin C, above 7 mg/100 g, was recorded in juice obtained from pomace (P1W and P2W), and the lowest in juice from fruit pulp subjected to mashing alone (P1).
In fresh blueberries, depending on the variety, the content of vitamin C varies from 15.2 to 22.7 mg/100 g and decreases to 4.2–9.1 mg/100 g after 12 months of storage in frozen state [8]. However, according to Sinelli et al. [33], the content of AA in highbush blueberry varies from 0.05 to 8.0 mg/100 g. In our experiment, irrespective of the treatment applied, total degradation of AA was noted, together with an increase in the content of DHAA. As shown by Friedrich [37], AA easily undergoes oxidation, transforming into DHAA.
Our study demonstrated an impact of preliminary treatment on the acidity of the resultant juices (Table 3). Juice produced according to P2 was characterized by the lowest acidity. In the case of the juices from pomace, a higher titratable acidity was obtained in the case of juice extracted according to P2W.
Conclusions
Our study indicates that the pressing efficiency and the quality of highbush blueberry juice depend on the preliminary treatment. The character of the pre-treatment has a significant effect on the content of lutein and vitamin C in the juice. Irrespective of the preliminary treatment, degradation of AA was observed, as was an increase in the content of DHAA in the juices. In terms of the energy consumption of the process, it is beneficial to extract highbush blueberry juice by pressing mashed fruits, followed by an additional pressing of pomace, subjected to freezing and thawing prior to the pressing. In this case, there are no costs related to freezing and thawing the pulp before it is pressed. There are only extra costs related to freezing and thawing the pomace. Nevertheless, juice extracted in this manner is characterized by a relatively low level of lutein and vitamin C. In terms of the nutritional value, the recommended method of highbush blueberry juice extraction consists in juice pressing from frozen and thawed fruits, and additionally from pomace subjected to treatment consisting in pomace freezing and thawing before the pressing. In this way, the highest total content of lutein and vitamin C was obtained. The method proposed permits the production of juices with health-promoting properties. It is recommended to conduct further research aimed at an estimation of the effect of the freezing technique and, possibly, of the time of storage of frozen fruits on the efficiency of pressing and on the content of lutein and AA and DHAA in the juice.
References
E. Murillo, J.A. Melendez-Martinez, F Portugal Screening of vegetables and fruits from Panama for rich sources of lutein and zeaxanthin. Food Chem. 122, 167–172 (2010)
A. Alves-Rodrigues, A. Shao, The science behind lutein. Toxicol. Lett. 150(1), 57–83 (2004)
R.L. Roberts, J. Green, B. Lewis, Lutein and zeaxanthin in eye and skin health. Clin. Dermatol. 27(2), 195–201 (2009)
D. Marinova, F. Ribarova, HPLC determination of carotenoids in Bulgarian berries. J. Food Compos. Anal. 20, 370–374 (2007)
K.A. Lashmanova, O.A. Kuzivanova, O.V. Dymova, Northern berries as a source of carotenoids. Acta Biochim. Pol. 59(1), 133–134 (2012)
K. Nyyssonen, J.T. Salonen, M.T. Parviainen, Ascorbic acid, in Modern Chromatographic Analysis of Vitamins, ed. by A.P. De Leenheer, W.E. Lambert, J.F. Van Bocxlaer (Marcel Dekker, New York, 2000), p. 287
M.W. Davey, M. Van Montagu, D. Inzé, M. Sanmartin, A. Kanellis, N. Smirnoff, I.J.J. Benzie, J.J. Strain, L.D. Favel, J. Fletcher, Plant l-ascorbic acid: chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 80, 825–860 (2000)
K. Skupień, Evaluation of chemical composition of fresh and frozen blueberry fruit (Vaccinium Corymbosum L.). Acta Sci. Pol. Hortorum Cultus 5(1), 19–25 (2006)
A. Michalska, G. Łysiak, Bioactive compounds of blueberries: post-harvest factors influencing the nutritional value of products. Int. J. Mol. Sci. 16, 18642–18663 (2015)
R.A. Moyer, K.E. Hummer, C.E. Finn, B. Frei, R.E. Wrolstad, Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: vaccinium, rubus, and ribes. J. Agric. Food Chem. 50, 519–525 (2002)
A.D.R. Castrejón, I. Eichholz, S. Roh, L.W. Kroh, S. Huyskens-Keil, Phenolic profile and antioxidant activity of highbush blueberry (Vaccinium corymbosum L.) during fruit maturation and ripening. Food Chem. 109, 564–572 (2008)
D. Li, X. Meng, B. Li, Profiling of anthocyanins from blueberries produced in China using HPLC-DAD-MS and exploratory analysis by principal component analysis. J. Food Compos. Anal. 47, 1–7 (2016)
L.R. Howard, C. Castrodale, C. Brownmiller, A. Mauromoustakos, Jam processing and storage effects on blueberry polyphenolics and antioxidant capacity. J. Agric. Food Chem. 58(7), 4022–4029 (2010)
V. Lohachoompol, G. Srzednicki, J. Craske, The change of total anthocyanins in blueberries and their antioxidant effect after drying and freezing. J. Biomed. Biotechnol. 5, 248–252 (2004)
C. Brownmiller, L.R. Howard, R.L. Prior, Processing and storage effects on monomeric anthocyanins, percent polymeric color, and antioxidant capacity of processed blueberry products. J. Food Sci. 73, 72–79 (2008)
J. Lee, R.W. Durst, R.E. Wrolstad, Impact of juice processing on blueberry anthocyanins and polyphenolics: comparison of two pretreatments. J. Food Sci. 67(5), 1660–1667 (2002)
S. Oancea, M. Stoia, D. Coman, Effects of extraction conditions on bioactive anthocyanin content of Vaccinium corymbosum in the perspective of food applications. Procedia Eng. 42, 489–495 (2012)
M. Rossia, E. Giussania, R. Morelli, R. Lo Scalzo, R.C. Nani, D. Torreggianic, Effect of fruit blanching on phenolics and radical scavenging activity of highbush blueberry juice. Food Res. Int. 36, 999–1005 (2003)
J.A. Rothwell, A. Medina-Remón, J. Pérez-Jiménez, V. Neveu, V. Knaze, N. Slimani, A. Scalbert, Effects of food processing on polyphenol contents: a systematic analysis using Phenol-Explorer data. Mol. Nutr. Food Res. 59, 160–170 (2015)
J. Markowski, A. Baron, J.-M. Le Quéer, W. Płocharski, Composition of clear and cloudy juices from French and Polish apples in relation to processing technology. LWT Food Sci. Technol. 62, 813–820 (2015)
J.D. Wightman, R.E. Wrolstad, Beta-glucosidase activity in juice processing enzymes based on anthocyanin analysis. J. Food Sci. 61, 544–547 (1996)
R.E. Wrolstad, J.D. Wightman, Glycosidase activity of enzyme preparations used in fruit juice processing. Food Technol. (Chicago) 48(11), 90, 92-94, 96, 98 (1994)
R. Nadulski, J. Grochowicz, P. Sobczak, Z. Kobus, M. Panasiewicz, K. Zawislak, J. Mazur, A. Starek, W. Żukowicz-Sobczak, Application of freezing and thawing to carrot (Dacus carita L.) juice extraction. Food Bioprocess Technol. 8(1), 218–227 (2015)
R. Nadulski, J. Skwarcz, A. Sujak, Z. Kobus, K. Zawiślak, A. Stój, J. Wyrostek, Effect of pre-tratment on pressing efficiency and properties of rhubarb (Rheum rhaponticum L.) juice. J. Food Eng. 166(1), 370–376 (2015)
PN-EN 12143:2000, Fruit and vegetable juices—estimation of soluble solids content—refractometric method (Warszawa, PKN)
PN-EN 12147:2000, Fruit and vegetable juices—determination of titratable acidity (Warszawa, PKN)
A. Mazurek, J. Jamroz, Precision of dehydroascorbic acid quantitation with the use of the subtraction method—validation of HPLC–DAD method for determination of total vitamin C in food. Food Chem. 173, 543–550 (2015)
R.C.H. Caldwell, J.S. Britz, Effect of supplemental ultraviolet radiation on the carotenoid and chlorophyll composition of green house-grown leaf lettuce (Lactuca sativa L.) culivars. J. Food Compos. Anal. 19, 637–644 (2006)
B. Mou, Genetic variation of beta-carotene and lutein contents in lettuce. J. Am. Soc. Hortic. Sci. 130(6), 870–876 (2005)
Statistica 12, StatSoft Inc., 2300 E. 14th St. Tulsa, OK74104, USA, www.StatSoft.com (2014)
P. Capek, C.M.G.C. Renard, J.F. Thibault, Enzymatic degradation of cell walls of apples and characterization of solubilized products. Int. J. Biol. Macromol. 17(6), 337–340 (1995)
D. Chevalier, A. Le Bai, M. Ghoul, Freezing and ice crystals formed in cylindrical food model: part I. Freezing at atmospheric pressure. J. Food Eng. 46, 277–285 (2000)
N. Sinelli, A. Spinardi, V. Di Egidio, I. Mignani, E. Casiraghi, Evaluation of quality and nutraceutical content of blueberries (Vaccinium corymbosum L.) by near and mid-infrared spectroscopy. Postharvest Biol. Technol. 50(1), 31–36 (2008)
K. Stewart, Processing in cranberry, blueberry, currant, and gooseberry, in Processing fruits; science and technology, ed. by L.P. Somogyi (Technomic Pub. Co, Lancaster, 1996)
A. Gryszczyńska, B. Gryszczyńska, B. Opala, Karotenoidy. Naturalne źródła, biosynteza, wpływ na organizm ludzki. Post. Fitoter. 2, 127–143 (2011)
A. Bunea, D. Rugină, A. Pintea, S. Andrei, C. Bunea, R. Pop, P. Bele, Carotenoid and fatty acid profiles of bilberries and cultivated blueberries from Romania. Chem. Pap. 66(10), 935–939 (2012)
W. Fridrich, Vitamins (Walter de Gruyter, Berlin), p. 1058. ISBN 3-11-010244-7/0-89925-273-7 (1988)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
Authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Nadulski, R., Masłowski, A., Mazurek, A. et al. Vitamin C and lutein content of northern highbush blueberry (Vaccinium corymbosum L.) juice processed using freezing and thawing. Food Measure 13, 2521–2528 (2019). https://doi.org/10.1007/s11694-019-00172-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-019-00172-x