Abstract
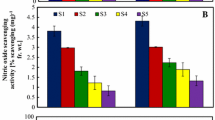
In this study Crocus sativus L. stigmas obtained from two ecogeographical zones of Jammu and Kashmir, India were evaluated for proximate composition and antioxidant assays. Protein, carbohydrate and caloric value were slightly higher in Kishtwar Jammu cultivar (J) while, moisture, fat and ash contents were higher in Pampore pulwama (K) cultivar but, the difference was non-significant (P > 0.05). In vitro antioxidant assays of methanolic extracts of the C. sativus stigma samples of the two cultivars was evaluated by total phenolic contents (TPC), 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity, reducing power, 2,2-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) radical scavenging activity (ABTS), ferric reducing antioxidant power (FRAP), ferrous ion-chelating potential (metal chelating activity)and the lipid peroxidation methods at different concentrations (100, 200 and 300 µg/mL). The results showed that TPC, ABTS, FRAP values were significantly higher in J cultivar (P < 0.05) while, DPPH, reducing power, ferrous ion-chelating potential and lipid peroxidation were slightly higher but the difference was non-significant (P > 0.05). The study concluded that saffron from Kishtwar Jammu showed strong antioxidant potential than pampore pulwama cultivar. Thus the selections of saffron from different ecogeographical zones of Jammu and Kashmir are heterogeneous.






Similar content being viewed by others
Abbreviations
- DPPH:
-
1,1-Diphenyl-2-picrylhydrazyl
- ABTS:
-
2,2-Azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid)
- TPTZ:
-
2,4,6-Tripyridyl-s-triazine
- FRAP:
-
Ferric reducing antioxidant power
- TPC:
-
Total phenolic content
- TBA:
-
Thiobarbituric acid
- BHT:
-
Butylated hydroxyl toluene
- MDA:
-
Malonyldialdehyde
References
P. Goldblatt, T.J. Davies, J.C. Manning, M. van der Bank, V. Savolainen, Hylogeny of Iridaceae subfamily Crocoideae based on a combined multigene plastid DNA analysis. Aliso 22, 399–411 (2006)
F. Gresta, Saffron, an alternative crop for sustainable agricultural systems. A review. Agron. Sustain. Dev. 28, 95–112 (2007)
R. Sánchez-Vioquea, M.F. Rodríguez-Condea, J.V. Reina-Urenaa, M.A. Escolano-Terceroa, D.O. Herraiz-Pe˜nalvera, M.A. Santana-Méridas, In vitro antioxidant and metal chelating properties of corm, tepal and leaf from saffron (Crocus sativus L.). Ind. Crops Prod. 39, 149–153 (2012)
C. Ulbricht, J. Conquer, D. Costa, W. Hollands, C. Iannuzzi, R. Isaac, J.K. Jordan, N. Ledesma, C. Ostroff, J.M. GrimesSerrano, M.D. Shaffer, M. Varghese, An evidence-based systematic review of saffron (Crocus sativus) by the natural standard research collaboration. J. Diet. 8, 58–114 (2011)
K. Sano, H. Himeno, In vitro proliferation of saffron (Crocus sativus L.) stigma. Plant Cell Tissue Organ Cult. 11, 159–166 (1987)
A.M. Husaini, M.A. Bhat, A.N. Kamili, M.A. Mir, Kashmir saffron in crisis. Curr. Sci. 104, 686–687 (2013)
M. Carmona, A. Zalacain, G.L. Alonso, The chemical composition of saffron: colour, taste and aroma, 1st edn. (Albacete, Bomarzo, 2006)
A. Gismondi, M. Serio, L. Canuti, A. Canini, Biochemical, antioxidant and antineoplastic properties of Italian saffron (Crocus sativus L.). Am. J. Plant Sci. 3, 1573–1580 (2012)
S.R. Sampathu, S. Shivashavikars, Y.S. Lewis, Saffron (Crocus sativus Linn.) cultivation, processing, chemistry and standardization. Crit. Rev. Food Sci. Nutr. 20, 123–157 (1984)
F.I. Abduyaev, Cancer chemopreventive and tumoricidal properties of saffron (Crocus sativus L.). Exp. Biol. Med. 227, 20–25 (2002)
J.A. Fernández, Biology, biotechnology and biomedicine of saffron. Recent Res. Dev. Plant Sci. 2, 127–159 (2004)
J.A. Fernández, Anticancer properties of saffron: Crocus sativus Linn. Lead molecules from natural products: discovery and new trends. Adv. Phytomed. 2, 313–330 (2006)
M. Ajami, S. Eghtesadi, H. Pazoki-Toroudi, R. Habibey, A.E. Soltan, Effect of Crocus sativus on gentamicin induced nephrotoxicity. Biol. Res. 43, 83–90 (2010)
C.D. Kanakis, P.A. Tarantilis, H.A. Tajmir Riahi, M.G. Polissiou, Crocetin, dimethylcrocetin, and safranal bind human serum albumin: stability and antioxidative properties. J. Agric. Food Chem. 55, 970–977 (2007)
S.A. Ordoudi, C.D. Befani, N. Nenadis, G.G. Koliakos, M.Z. Tsimidou, Further examination of antiradical properties of Crocus sativus stigmas rich in crocins. J. Agric. Food Chem. 57, 3080–3086 (2009)
E. Moshiri, A.A. Basti, A.A. Noorbala, A.H. Jamshidi, S.H. Abbasi, S. Akhondzadeh, Crocus sativus L. (petal) in the treatment of mild-to-moderate depression: a double-blind, randomized and placebo-controlled trial. Phytomed. 13, 607–611 (2006)
H. Hosseinzadeh, H.H.M. Younesi, Antinocicetive and anti-inflammatory effects of Crocus sativus L. stigma and petals extracts in mice. BMC Pharmacol. 2, 1–8 (2002)
C.Y. Li, E.J. Lee, T.S. Wu, Antityrosinase principles and constituents of the petals of Crocus sativus. J. Nat. Prod. 67, 437–440 (2004)
S.A.H. Goli, F. Mokhtari, R. Mehdi, Phenolic compounds and antioxidant activity from saffron (Crocus sativus L.) petal. J. Agric. Sci. 4, 2010–2012 (2012)
J.A. Fernandez, Biology, biotechnology and biomedicine of saffron. Recent Res. Dev. Plant Sci. 2, 127–159 (2004)
AOAC, Official methods of analysis, 16th edn. (Association of Official Analytical Chemists, Arlington, 1995)
M.N. Sato, S. Fujwara, H. Tsuchiya, T. Fujii, M. Linuma, H. Tosa, Y. Ohkaw, Flavones with antibacterial activity against carcinogenic bacteria. J. Ethnopharmocol. 54, 171–176 (1996)
M. Joyeux, A. Lobstein, F. Mortier, Comparative antilipoperoxidant, antinecrotic and scavenging properties of terpenes and biflavones from Gingko and some flavonoids. Planta Med. 61, 126–129 (1995)
G.C. Yen, H.Y. Chen, Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 43, 27–32 (1995)
H.E. Muller, Detection of hydrogen peroxide produced by microorganism on ABTS peroxidase medium. Zentralblatt fur Bakteriol. Mikrobiol. Hyg. 259, 151–158 (1985)
I.F. Benzie, J.J. Strain, The ferric reducing ability of plasma (FRAP) as a measure of ‘‘antioxidant power”: the FRAP assay. Anal. Biochem. 239, 70–76 (1996)
R. Pulido, L. Bravo, F. Saura-Calixto, Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 48, 3396–3402 (2000)
P. Carter, Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine). Anal. Biochem. 40, 450–458 (1971)
T. Osama, M. Namiki, A novel type of antioxidant isolated from leaf wax of Eucalyptus leaves. Agric. Biol. Chem. 45, 735–739 (1981)
ISO 3632, Saffron (Crocus sativus L.). Part 1: specification, part 2: test methods (International Organization for Standardization, Geneva, 2011)
L.N. Bell, Moisture effects on food’s chemical stability, in Water activity in foods: fundamentals and applications, ed. by G.V. Barbosa-Ca´no-vas, A.J. Fontana, S.J. Schmidt, T.P. Labuza (Blackwell Publishing Ltd, Oxford, 2008), pp. 173–198
DRIs, Dietary references intakes: food and nutrition board, institute of medicine. (National Academies of Sciences, National Academy Press, 2012). http://ods.odih.gov/health_information/dietary_reference_intakes.aspx. 15 May 2012
J. Serrano-Dıaz, A.M. Sanchez, M. Martınez-Tome´, P. Peter Winterhalter, G.L.A. Alonso, A contribution to nutritional studies on Crocus sativus flowers and their value as food. J. Food Compos. Anal. 31, 101–108 (2013)
Y.S. Lewis, S.R. Sampathu, S. Shivashankar, C.P. Natarajan, Quality evaluation of saffron. Ind. Arecanutn Spices Cacoa J. 4, 113–115 (1981)
A. Othman, A. Ismail, N. Abdul Ghani, I. Adenan, Antioxidant capacity and phenolic content of cocoa beans. Food Chem. 100, 1523–1530 (2007)
R. Sariri, R. Sabbaghzadeh, F. Poumohamad, In-vitro antioxidant and anti-tyrosinase activity of methanol extracts from Crocus sativus flowers. Pharmacologyonline 3, 1–11 (2011)
E. Karimi, E. Oskoueian, R. Hendra, H.Z.E. Jaafar, Evaluation of Crocus sativus L. stigma phenolic and flavonoid compounds and its antioxidant activity. Molecules 15, 6244–6256 (2010). doi:10.3390/molecules15096244
I.C. Jang, J.H. Park, E.J. Park, H.R. Park, S.C. Lee, Antioxidative and antigenotoxic activity of extracts from cosmos (Cosmos bipinnatus) flowers. Plant Foods Hum. Nutr. 63, 205–210 (2008)
K. Shimada, K. Fujikawa, K. Yahara, T. Nakamura, Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 40, 945–948 (1992)
S. Rahaiee, S. Moini, M. Hashemi, S.A. Shojaosadati, Evaluation of antioxidant activities of bioactive compounds and various extracts obtained from saffron (Crocus sativus L.). J. Food Sci. (2014). doi:10.1007/s13197-013-1238-x
V. Katalinic, S.S. Mozina, I. Generalic, D. Skroza, I. Ljubenkov, A. Klancnik, Phenolic profile, antioxidant capacity, and antimicrobial activity of leaf extracts from six Vitis vinifera L. varieties. Int. J. Food Prop. 16(1), 45–60 (2014)
K. Jomova, M. Valko, Advances in metal-induced oxidative stress and human disease. Toxicology 283, 65–87 (2011)
G. Acar, N.M. Dogan, M.E. Duru, I. Kıvrak, Phenolic profiles, antimicrobial and antioxidant activity of the various extracts of Crocus species in Anatolia. Afr. J. Microb. Res. 4(11), 1154–1161 (2010)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muzaffar, S., Rather, S.A., Khan, K.Z. et al. Nutritional composition and in-vitro antioxidant properties of two cultivars of Indian saffron. Food Measure 10, 185–192 (2016). https://doi.org/10.1007/s11694-015-9292-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-015-9292-x