Abstract
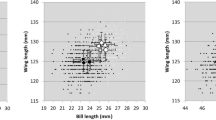
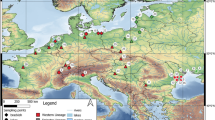
Geographically structured variation in morphology is a common phenomenon in animals with environmental factors covarying with both latitude and biogeographic barriers having profound impacts on body size and shape. The Pacific blue-eye (Pseudomugil signifer) is a freshwater fish that lives along Australia’s east coast and occurs on either side of a terrestrial barrier, the Burdekin Gap. By quantifying the size and shape of males and females from 10 populations we found that Pacific blue-eyes are not sexually size dimorphism north of the Burdekin Gap whereas the degree of dimorphism was dependent upon latitude south of the barrier. Rensch’s rule was not supported as the degree of male-biased size dimorphism did not increase with increasing population mean body size. Body shape was related to body size and was sexually dimorphic south of the Burdekin Gap but not north of it. Our study represents a rare case of identifying how both body size and shape differ with respect to latitude and a major terrestrial biogeographic barrier and lends further support to the notion that P. signifer may comprise two species, or incipient species, that are separated by the Burdekin Gap.









Similar content being viewed by others
References
Abell, A., Cole, B., Reyes, R., & Wiernasz, D. (1999). Sexual selection on body size and shape in the western harvester ant, Pogonomyrmex occidentalis cresson. Evolution, 53, 535–545.
Adams, D. C., & Church, J. O. (2008). Amphibians do not follow Bergmann’s rule. Evolution, 62, 413–420.
Adams, D. C., & Church, J. O. (2011). The evolution of large-scale body size clines in Plethodon salamanders: Evidence of heat-balance or species-specific artifact? Ecography, 34, 1067–1075.
Adams, D. C., & Collyer, M. L. (2007). Analysis of character divergence along environmental gradients and other covariates. Evolution, 61, 510–515.
Adams, D. C., & Collyer, M. L. (2009). A general framework for the analysis of phenotypic trajectories in evolutionary studies. Evolution, 63, 1143–1154.
Adams, D. C., & Otarola-Castillo, E. (2012). Geomorph: Software for geometric morphometric analyses. R package version 1.1-0. http://cran.r-project.org/web/packages/geomorph/index.html.
Adams, D. C., & Otarola-Castillo, E. (2013). Geomorph: An R package for the collection and analysis of geometric morphometric shape data. Methods in Ecology and Evolution (in press).
Adams, D. C., & Nistri, A. (2010). Ontogenetic convergence and evolution of foot morphology in European cave salamanders (Family: Plethodontidae). BMC Evol Biol, 10.
Adams, D. C., Rohlf, F. J., & Slice, D. E. (2004). Geometric morphometrics: Ten years of progress following the “revolution”. Italian Journal of Zoology, 71, 5–16.
Allen, G., Midgley, S., & Allen, M. (2003). Field guide to the freshwater fishes of Australia. Collingwood, Vic: CSIRO Publishing.
Anderson, M., & ter Braak, C. (2003). Permutation tests for multi-factorial analysis of variance. Journal of Statistical Computation and Simulation, 73, 85–113.
Angilletta, M., & Dunham, A. (2003). The temperature-size rule in ectotherms: Simple evolutionary explanations may not be general. The American Naturalist, 162, 332–342.
Belk, M. C., & Houston, D. D. (2002). Bergmann’s rule in ectotherms: A test using freshwater fishes. The American Naturalist, 160, 803–808.
Bergmann, C. (1847). Über die verhältnisse der wärmeökonomie der thiere zu ihrer grösse. Göttinger Studien, 3, 595–708.
Berns, C. M., & Adams, D. C. (2010). Bill shape and sexual shape dimorphism between two species of temperate hummingbirds: Black-chinned hummingbird (Archilochus alexandri) and ruby-throated hummingbird (A. colubris). The Auk, 127, 626–635.
Blanckenhorn, W. (2005). Behavioral causes and consequences of sexual size dimorphism. Ethology, 111, 977–1016.
Blanckenhorn, W. U., & Demont, M. (2004). Bergmann and converse Bergmann latitudinal clines in arthropods: Two ends of a continuum? Integrative and Comparative Biology, 44, 413–424.
Blanckenhorn, W. U., Stillwell, R. C., Young, K. A., Fox, C. W., & Ashton, K. G. (2006). When Rensch meets Bergmann: Does sexual size dimorphism change systematically with latitude? Evolution, 60, 2004–2011.
Bookstein, F. L. (1986). Size and shape spaces for landmark data in two dimensions. Statistical Science, 1, 181–222.
Bookstein, F. L. (1997). Landmark methods for forms without landmarks: Morphometrics of group differences in outline shape. Medical Image Analysis, 1, 225–243.
Bookstein, F., Schäfer, K., Prossinger, H., Seidler, H., Fieder, M., Stringer, C., et al. (1999). Comparing frontal cranial profiles in archaic and modern homo by morphometric analysis. Anatomical Record Part B, New Anatomist, 257, 217–224.
Brown, M., Cooksley, H., Carthew, S. M., & Cooper, S. J. B. (2006). Conservation units and phylogeographic structure of an arboreal marsupial, the yellow-bellied glider (Petaurus australis). Australian Journal of Zoology, 54, 305–317.
Burns, J. G., Di Nardo, P., & Rodd, F. H. (2009). The role of predation in variation in body shape in guppies Poecilia reticulata: A comparison of field and common garden phenotypes. Journal of Fish Biology, 75, 1144–1157.
Butler, M. A., & Losos, J. (2002). Multivariate sexual dimorphism, sexual selection, and adaptation in Greater Antillean Anolis lizards. Ecological Monographs, 72, 541–559.
Butler, M. A., Sawyer, S. A., & Losos, J. B. (2007). Sexual dimorphism and adaptive radiation in Anolis lizards. Nature, 447, 202–205.
Chapple, D. G., Hoskin, C. J., Chapple, S. N., & Thompson, M. B. (2011). Phylogeographic divergence in the widespread delicate skink (Lampropholis delicata) corresponds to dry habitat barriers in eastern Australia. BMC Evolutionary Biology, 11, 191.
Claude, J. (2008). Morphometrics with R. Springer Verlag.
Collyer, M. L., & Adams, D. C. (2007). Analysis of two-state multivariate phenotypic change in ecological studies. Ecology, 88, 683–692.
Drake, A. G., & Klingenberg, C. P. (2008). The pace of morphological change: Historical transformation of skull shape in St Bernard dogs. Proceedings of the Royal Society B: Biological Sciences, 275, 71–76.
Endler, J. (1995). Multiple-trait coevolution and environmental gradients in guppies. Trends in Ecology & Evolution, 10, 22–29.
Fairbairn, D. J. (1997). Allometry for sexual size dimorphism: Pattern and process in the coevolution of body size in males and females. Annual Review of Ecology and Systematics, 28, 659–687.
Fairbairn, D. J. (2005). Allometry for sexual size dimorphism: Testing two hypotheses for Rensch’s rule in the water strider Aquarius remigis. The American Naturalist, 166, S69–S84.
Fairbairn, D. J., & Preziosi, R. (1994). Sexual selection and the evolution of allometry for sexual size dimorphism in the water strider, Aquarius remigis. The American Naturalist, 144, 101–118.
Felsenstein, J. (2002). Quantitative characters, phylogenies and morphometrics. In N. MacLeod & P. Forey (Eds.), Morphology, Shape and Phylogeny (pp. 27–44). Bova Raton, FL: CRC Press.
Fitzpatrick, B. M. (2012). Underappreciated consequences of phenotypic plasticity for ecological speciation. International Journal of Ecology, 2012, 1–12.
Georga, I., & Koumoundouros, G. (2010). Thermally induced plasticity of body shape in adult zebrafish Danio rerio (Hamilton, 1822). Journal of Morphology, 271, 1319–1327.
Hadfield, A., Ivantsoff, V., & Johnson, P. (1979). Clinal variation in electrophoretic and morphological characters between two nominal species of the genus Pseudomugil (Pisces: Atheriniformes: Pseudomugilidae). Marine & Freshwater Research, 30, 375–386.
Hendry, A., Kelly, M. L., Kinnison, M. T., & Reznick, D. N. (2006). Parallel evolution of the sexes? Effects of predation and habitat features on the size and shape of wild guppies. Journal of Evolutionary Biology, 19, 741–754.
Herczeg, G., Gonda, A., & Merilä, J. (2010). Rensch’s rule inverted—female-driven gigantism in nine-spined stickleback Pungitius pungitius. Journal of Animal Ecology, 79, 581–588.
Joseph, L., & Moritz, C. (1994). Mitochondrial DNA phylogeography of birds in eastern Australian rainforests: First fragments. Australian Journal of Zoology, 42, 385–403.
Kaliontzopoulou, A., Adams, D. C., Meijden, A., Perera, A., & Carretero, M. A. (2012). Relationships between head morphology, bite performance and ecology in two species of Podarcis wall lizards. Evolutionary Ecology, 26, 825–845.
Kelly, C. D., & Adams, D. C. (2010). Sexual selection, ontogenetic acceleration, and hypermorphosis generates male trimorphism in Wellington tree weta. Evolutionary Biology, 37, 200–209.
Kelly, C. D., Bussiere, L. F., & Gwynne, D. (2008). Sexual selection for male mobility in a giant insect with female-biased size dimorphism. The American Naturalist, 172, 417–423.
Langerhans, R., & DeWitt, T. (2004). Shared and unique features of evolutionary diversification. The American Naturalist, 164, 335–349.
Langerhans, R., Layman, C., Shokrollahi, A., & DeWitt, T. (2004). Predator-driven phenotypic diversification in Gambusia affinis. Evolution, 58, 2305–2318.
Lengkeek, W., Didderen, K., Cote, I. M., van der Zee, E. M., Snoek, R. C., & Reynolds, J. D. (2008). Plasticity in sexual size dimorphism and Rensch’s rule in Mediterranean blennies (Blenniidae). Canadian Journal of Zoology, 86, 1173–1178.
McGlashan, D., & Hughes, J. (2002). Extensive genetic divergence among populations of the Australian freshwater fish, Pseudomugil signifer (Pseudomugilidae), at different hierarchical scales. Marine Freshwater Research.
Outomuro, D., & Johansson, F. (2011). The effects of latitude, body size, and sexual selection on wing shape in a damselfly. Biological Journal of the Linnean Society, 102, 263–274.
Pusey, B., Kennard, M., & Arthington, A. (2004). Freshwater fishes of North-Eastern Australia. Collingwood, Vic: Csiro Publishing.
Rensch, B. (1960). Evolution above the species level. New York: Columbia University Press.
Rohlf, F. J. (2010). tpsRelw: Relative warps analysis.
Rohlf, F. J., & Marcus, L. (1993). A revolution in morphometrics. Trends in Ecology & Evolution, 8, 129–132.
Rohlf, F. J., & Slice, D. E. (1990). Extensions of the procrustes method for the optimal superimposition of landmarks. Systematic Zoology, 39, 40–59.
Schäuble, C. (2004). Variation in body size and sexual dimorphism across geographical and environmental space in the frogs Limnodynastes tasmaniensis and L. peronii. Biological Journal of the Linnean Society, 82, 39–56.
Serb, J. M., Alejandrino, A., Otarola-Castillo, E., & Adams, D. C. (2011). Morphological convergence of shell shape in distantly related scallop species (Mollusca: Pectinidae). Zoological Journal of the Linnean Society, 163, 571–584.
Stone, G. N., Nee, S., & Felsenstein, J. (2011). Controlling for non-independence in comparative analysis of patterns across populations within species. Philosophical Transactions of the Royal Society B: Biological SciencesB, 366, 1410–1424.
Szekely, T., Freckleton, R., & Reynolds, J. (2004). Sexual selection explains Rensch’s rule of size dimorphism in shorebirds. Proceedings of the National Academy of Sciences of the United States of America, 101, 12224–12227.
Teder, T., & Tammaru, T. (2005). Sexual size dimorphism within species increases with body size in insects. Oikos, 108, 321–334.
Unmack, P. (2001). Biogeography of Australian freshwater fishes. Journal of Biogeography, 28, 1053–1089.
Verhoeven, K. J. F., Simonsen, K. L., & McIntyre, L. M. (2005). Implementing false discovery rate control: Increasing your power. Oikos, 108, 643–647.
Wiley, E. (1988). Parsimony analysis and vicariance biogeography. Systematic Zoology, 37, 271–290.
Wong, B. (2004). Superior fighters make mediocre fathers in the Pacific blue-eye fish. Animal Behaviour, 67, 583–590.
Wong, B., Keogh, J., & Jennions, M. D. (2004a). Mate recognition in a freshwater fish: Geographical distance, genetic differentiation, and variation in female preference for local over foreign males. Journal of Evolutionary Biology, 17, 701–708.
Wong, B., Keogh, J., & McGlashan, D. (2004b). Current and historical patterns of drainage connectivity in eastern Australia inferred from population genetic structuring in a widespread freshwater fish Pseudomugil signifer (Pseudomugilidae). Molecular Ecology, 13, 391–401.
Young, K. A. (2005). Life-history variation and allometry for sexual size dimorphism in Pacific salmon and trout. Proceedings of the Royal Society of London. Series B: Biological Sciences, 272, 167–172.
Zelditch, M., Swiderski, D., Sheets, H., & FINK, W. (2004). Geometric morphometrics for biologists. London: Academic Press.
Acknowledgments
We thank Mark McGrouther (Ichthyology, Collection Manager) for access to preserved P. signifer at the Australian Museum and Hugh Spencer (Cape Tribulation Tropical Research Station) for advice and hospitality and two anonymous referees for their valuable input. Fish were collected under a Queensland General Fisheries Permit. This work was supported by an A.N.U. Faculty of Science Research Grant and Iowa State University faculty start-up funds to CDK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kelly, C.D., Folinsbee, K.E., Adams, D.C. et al. Intraspecific Sexual Size and Shape Dimorphism in an Australian Freshwater Fish Differs with Respect to a Biogeographic Barrier and Latitude. Evol Biol 40, 408–419 (2013). https://doi.org/10.1007/s11692-013-9224-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-013-9224-9