Abstract
Purpose
This study aimed to analyze the frequency of piroplasmids in the blood of dogs in Rio de Janeiro, compare the performance of microscopic techniques, assess the risk factors associated with infections and also molecularly and morphologically characterize the piroplasmids identified.
Methods
In all, 407 blood samples were collected from dogs between 2018 and 2019. These were subjected to microscopic parasitological techniques for thin and thick smears, stained with Giemsa and using a rapid staining kit. The slides were read under an optical microscope and the protozoa were characterized morphometrically. In addition, the blood samples were subjected to molecular characterization for diagnosing piroplasmid species using primers that amplified the gene 18S rRNA.
Results
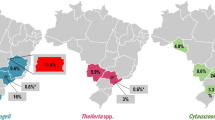
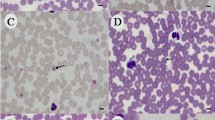
Piroplasmids were detected in 38 (9.3%) samples. Of these, 33 samples presented nucleotide sequences compatible with Babesia vogeli. Most of the positive samples were young, male, defined breeds dogs that had been attended in clinics in São Gonçalo city. Thrombocytopenia and leukopenia were the hematological alterations more observed in positive samples, but positive samples without alterations were also detected. The sex was the only variable that showed statistical differences. Males dogs being more often infected than females (p < 0.05). The microscope slides mostly showed piriform and oval merozoites measuring greater than 2.5 µm in length, which were compatible with B. vogeli. However, smaller forms were also identified, thus demonstrating the polymorphic nature of this parasite.
Conclusion
Babesia vogeli was detected in blood samples from dogs in the metropolitan cities of Rio de Janeiro by molecular techniques in different parasite morphotypes.



Source: author’s photos
Similar content being viewed by others
References
Bilić P, Kuleš J, Rafaj BR, Mrljak V (2018) Canine babesiosis: where do we stand? Acta Vet-Beograd 2(68):127–160. https://doi.org/10.2478/acve-2018-0011
Laha R, Das M, Sen A (2015) Morphology, epidemiology, and phylogeny of Babesia: an overview. Trop Parasitol 5(2):94–100. https://doi.org/10.4103/2229-5070.162490
Hauschild S, Shayan P, Schein E (1995) Characterization and comparison of merozoite antigens of different Babesia canis isolates by serological and immunological investigations. Parasitol Res 81:638–642. https://doi.org/10.1007/BF00931839
Birkenheuer AJ, Neel J, Ruslander D, Levy MG, Breitschwerdt EB (2004) Detection and molecular characterization of a novel large Babesia species in a dog. Vet Parasitol 124(3–4):151–160. https://doi.org/10.1016/j.vetpar.2004.07.008
Kjemtrup AM, Wainwright K, Miller M, Penzhorn BL, Carreno RA (2006) Babesia conradae, sp. nov., a small canine Babesia identified in California. Vet Parasitol 1–2(138):103–111. https://doi.org/10.1016/j.vetpar.2006.01.044
Criado-Fornelio A, Martinez-Marcos A, Buling-Saraña A, Barba-Carretro A (2003) Molecular studies on Babesia, Theileria and Hepatozoon in Southern Europe: part I. Epizootiological aspects. Vet Parasitol 113(3–4):189–201. https://doi.org/10.1016/s0304-4017(03)00078-5
Baneth G, Florin-Christensen M, Cardoso L, Schnittger L (2015) Reclassification of Theileria annae as Babesia vulpes sp. nov. Parasit Vectors 8:207. https://doi.org/10.1186/s13071-015-0830-5
Baneth G, Cardoso L, Brilhante-Simões P, Schnittger L (2019) Establishment of Babesia vulpes n. sp. (Apicomplexa: Babesiidae), a piroplasmid species pathogenic for domestic dogs. Parasit Vectors 12(1):129. https://doi.org/10.1186/s13071-019-3385-z
Criado A, Martinez J, Buling A, Barba JC, Merino S, Jefferies R, Irwin PJ (2006) New data on epizootiology and genetics of piroplasms based on sequences of small ribosomal subunit and cytochrome b genes. Vet Parasitol 142(3–4):238–247. https://doi.org/10.1016/j.vetpar.2006.07.004
Trapp SM, Dagnone AS, Vidotto O, Freire RL, Amude AM, de Morais HS (2006) Seroepidemiology of canine babesiosis and ehrlichiosis in a hospital population. Vet Parasitol 140(3–4):223–230. https://doi.org/10.1016/j.vetpar.2006.03.030
Maggi RG, Krämer F (2019) A review on the occurrence of companion vector-borne diseases in pet animals in Latin America. Parasit Vectors 12(1):145. https://doi.org/10.1186/s13071-019-3407-x (PMID: 30917860)
Da Silva AS, França RT, Costa MM, Paim CB, Paim FC, Dornelles GL, Soares JF, Labruna MB, Mazzanti CM, Monteiro SG, Lopes STA (2011) Experimental infection with Rangelia vitalii in dogs: acute phase, parasitemia, biological cycle, clinical-pathological aspects and treatment. Exp Parasitol 128(4):347–352. https://doi.org/10.1016/j.exppara.2011.04.010
Soares JF, Girotto A, Brandão PE, Da Silva AS, França RT, Lopes ST, Labruna MB (2011) Detection and molecular characterization of a canine piroplasm from Brazil. Vet Parasitol 180(3–4):203–208. https://doi.org/10.1016/j.vetpar.2011.03.024
Lemos TD, Toma HK, Assad RQ, Silva AV, Corrêa RGB, Almosny NRP (2017) Clinical and hematological evaluation of Rangelia vitalii-naturally infected dogs in southeastern Brazil. Rev Bras Parasitol Vet 26(3):307–313. https://doi.org/10.1590/s1984-29612017040
Duarte SC, Louly CCB, Neto OJS, Romanowski TNA, Lino Junior RS, Linhares GFC (2008) Diagnóstico parasitológico e molecular da babesiose canina na cidade de Goiânia-GO. Rev Patol Trop 37(3):229–236. https://doi.org/10.5216/rpt.v37i3.5064
Lemos TD, Cerqueira AMF, Toma HK, Silva AV, Corrêa RGB, Paludo GR, Massard CL, Almosny NRP (2012) Detection and molecular characterization of piroplasms species from naturally infected dogs in southeast Brazil. Rev Bras Parasitol Vet 21(2):137–142. https://doi.org/10.1590/S1984-29612012000200012
Loretti AP, Barros SS (2004) Parasitismo por Rangelia vitalii em cães (“nambiuvú” “peste de sangue”) uma revisão crítica sobre o assunto. Arq Inst Biol 71(1):101–131
Santos FB, Gazeta GS, Correa LL, Lobão LF, Palmer JP, Dib LV, Damasceno JAL, Moura-Martiniano NO, Bastos OMP, Uchôa CMA, Barbosa AS (2020) Molecular evaluation of piroplasms and hematological changes in canine blood stored in a clinical laboratory in Niterói, Rio de Janeiro. Braz J Vet Parasitol 3:e012420. https://doi.org/10.1590/S1984-29612020057
Rizzi TE, Meinkoth JH, Clinkenbeard KD (2010) Normal hematology of the dog. In: Weiss DJ, Wardrop KJ (eds) Schalm’s veterinary hematology, 6th edn. Wiley-Blackwell, Ames
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33(1):159–174. https://doi.org/10.2307/2529310 (PMID: 843571)
Boozer AL, Macintire DK (2003) Canine babesiosis. Vet Clin North Am Small Anim Pract 33(4):885–904. https://doi.org/10.1016/s0195-5616(03)00039-1
Solano-Galego L, Sainz A, Roura X, Estrada-Pena A, Miró GA (2016) Review of canine babesiois: the European perspective. Parasit Vectors 9:1–18. https://doi.org/10.1186/s13071-016-1596-0
Araujo AC, Silveira JAG, Azevedo SS, Nieri-Bastos FA, Ribeiro MFB, Labruna MB, Horta MC (2015) Babesia canis vogeli infection in dogs and ticks in the semiarid region of Pernambuco, Brazil. Pesq Vet Bras 35(5):456–461. https://doi.org/10.1590/S0100-736X2015000500012
Silva VC, Lima ER, Dias MBMC, Fukahori FLP, Rêgo MAS, Júnior JWP, Kim PCP, Leitão RSCS, Mota RA, Carieli EPO (2016) Parasitological and molecular detection of Babesia canis vogeli in dogs of Recife, Pernambuco and evaluation of risk factors associated. Semina Ciên Agrár 37(1):163–172. https://doi.org/10.5433/1679-0359.2016v37n1p163
Duarte SC, Parente JP, Pereira M, Soares CM, Linhares GFC (2011) Phylogenetic characterization of Babesia canis vogeli in dogs in the state of Goias, Brazil. Rev Bras Parasitol Vet 20(4):274–280. https://doi.org/10.1590/S1984-29612011000400004
Harvey TV, Veloso JF, Santos RS, Assunção MS, Sauer L, Guedes PEB, Oliveira TNA, Albuquerque GR, Silva FL, Munhoz AD, Carlos RSA (2017) Babesia spp. and Ehrlichia chaffeensis infection in dogs from southeastern Bahia, Brazil. Acta Sci Vet 45(1457):1–9
Silva MCA, Mundim AV, Mendonça GA, Mundim MJS, Guimarães EC (2014) Hemoparasitos em cães domésticos naturalmente infectados, provenientes das zonas urbana e rural do município de Abadia dos Dourados, Minas Gerais, Brasil. Biosci J 30:892–900
Odwyer LH, Lopes VVA, Rubini AS, Paduan KS, Ribolla PEM (2009) Babesia spp. infection in dogs from rural areas of São Paulo State, Brazil. Rev Bras Parasitol Vet 18(2):23–26. https://doi.org/10.4322/rbpv.01802005
Miranda FJB, Albernaz AP, Melo OA Jr, Machado JA (2008) Freqüência de cães infectados por Babesia spp. em Campos dos Goytacazes, RJ. Ciên Anim Bras 9(1):238–241. https://www.revistas.ufg.br/vet/article/view/1030
Castro VV, Ayres ECBS, Canei DH, Pereira ME, Sousa VRF, Chitarra CS, Dutra V, Nakazato L, de Almeida ABPF (2020) Molecular prevalence and factors associated with Babesia vogeli infection in dogs in the Cerrado Mato-Grossense region of Brazil. Cienc Rural 50(2):e20190389. https://doi.org/10.1590/0103-8478cr20190389
Moraes PH, Rufino CP, Baraúna AR, Reis T, Agnol LT, Meneses AM, Aguiar DC, Nunes MR, Gonçalves EC (2015) Molecular characterization of Babesia vogeli in dogs from Belém, northern Brazil. Genet Mol Res 14(4):16364–16371. https://doi.org/10.4238/2015.December.9.4 (PMID: 26662431)
Barbosa COS, Garcia JR, de Melo Nasser Fava N, Pereira DA, Cunha MJR, Nachum-Biala Y, Cury MC, Baneth G (2020) Babesiosis caused by Babesia vogeli in dogs from Uberlândia State of Minas Gerais, Brazil. Parasitol Res 119(3):1173–1176. https://doi.org/10.1007/s00436-019-06515-3
Dantas-Torres F, Figueiredo LA (2006) Canine babesiosis: a Brazilian perspective. Vet Parasitol 141(3–4):197–203. https://doi.org/10.1016/j.vetpar.2006.07.030
Taboada J, Merchant SR (1991) Babesiosis of companion animals and man. Vet Clin North Am Small Anim Pract 21(1):103–123. https://doi.org/10.1016/s0195-5616(91)50011-5 (PMID: 2014615)
Spolidorio MG, Torres MM, Campos WNS, Melo ALT, Igarashi M, Amude AM, Labruna MB, Aguiar DM (2011) Molecular detection of Hepatozoon canis and Babesia canis vogeli in domestic dogs from Cuiabá, Brazil. Rev Bras Parasitol Vet 20(3):253–255. https://doi.org/10.1590/S1984-29612011000300015 (PMID: 21961759)
Sousa KCM, Fernandes MP, Herrera HM, Freschi CR, Machado RZ, André MR (2017) Diversity of piroplasmids among wild and domestic mammals and ectoparasites in Pantanal wetland, Brazil. Ticks Tick Borne Dis 9(2):245–253. https://doi.org/10.1016/j.ttbdis.2017.09.010 (PMID: 28941935)
Passos LM, Geiger SM, Ribeiro MF, Pfister K, Zahler-Rinder M (2005) First molecular detection of Babesia vogeli in dogs from Brazil. Vet Parasitol 127(1):81–85. https://doi.org/10.1016/j.vetpar.2004.07.028
Li XW, Zhang XL, Huang HL, Li WJ, Wang SJ, Huang SJ, Shao JW (2020) Prevalence and molecular characterization of Babesia in pet dogs in Shenzhen, China. Comp Immunol Microbiol Infect Dis 70:101452. https://doi.org/10.1016/j.cimid.2020.101452 (Epub 2020 Feb 21. PMID: 32120143)
Oyamada M, Davoust B, Boni M, Dereure J, Bucheton B, Hammad A, Itamoto K, Okuda M, Inokuma H (2005) Detection of Babesia canis rossi, B. canis vogeli, and Hepatozoon canis in dogs in a village of eastern Sudan by using a screening PCR and sequencing methodologies. Clin Diag Lab Immunol 12(11):1343–1346. https://doi.org/10.1128/CDLI.12.11.1343-1346.2005
Criado-Fornelio A, Rey-Valeiron C, Buling A, Barba-Carretero JC, Jefferies R, Irwin P (2007) New advances in molecular epizootiology of canine hematic protozoa from Venezuela, Thailand and Spain. Vet Parasitol 144(3–4):261–269. https://doi.org/10.1016/j.vetpar.2006.09.042 (PMID: 17088022)
Di Cataldo S, Ulloa-Contreras C, Cevidanes A, Hernández C, Millán J (2002) Babesia vogeli in dogs in Chile. Transbound Emerg Dis. https://doi.org/10.1111/tbed.13609 (Epub ahead of print. PMID: 32367669)
Rey-Valeiron C, Criado-Fornelio A, Zavala E, Granados R (2007) Parasitological and molecular characterization of a Venezuelan isolate of Babesia canis. Rev Cient (Maracaibo) 17(1):21–27
Benigno RNM, Rodrigues BRS, Serra-Freire NM (2011) Avaliação das infecções por Babesia e Ehrlichia em cães e das infecções humanas por carrapatos oriundos desses cães no município de Campinas, Estado de São Paulo. Rev Bras Med Vet 33(4):238–245
Costa-Júnior LM, Ribeiro MF, Rembeck K, Rabelo EM, Zahler-Rinder M, Hirzmann J, Pfister K, Passos LM (2009) Canine babesiosis caused by Babesia canis vogeli in rural areas of the State of Minas Gerais, Brazil and factors associated with its seroprevalence. Res Vet Sci 86(2):257–260. https://doi.org/10.1016/j.rvsc.2008.07.002 (Epub 2008 Aug 23. PMID: 18723199)
Imre M, Farkas R, Ilie MS, Imre K, Dărăbuş G (2013) Survey of babesiosis in symptomatic dogs from Romania: occurrence of Babesia gibsoni associated with breed. Ticks Tick Borne Diseases 4(6):500–502
Silva AB, Costa PA, Sá JC, Costa FB, Dos Santos AC, Guerra RMSNC (2012) Detecção molecular de Babesia canis vogeli em cães e em Rhipicephalus sanguineus na mesoregião do Oeste Maranhense, Nordeste Brasileiro. Ciên Anim Bras 13(3):388–395. https://doi.org/10.5216/cab.v13i3.18439
Guimarães AM, Rocha MBM, Oliveira TMFS, Rosado IR, Morais LG, Santos RRD (2009) Fatores associados à soro positividade para Babesia, Toxoplasma, Neospora e Leishmania em cães atendidos em nove clínicas veterinárias do município de Lavras, MG. Rev Bras Parasitol Vet 18:49–53. https://doi.org/10.4322/rbpv.018e1009
IBGE–Instituto Brasileiro de Geografia e Estatística. https://cidades.ibge.gov.br/brasil/rj/sao-goncalo/panorama. Accessed 09 Sept 2019.
Dantas-Torres F (2010) Biology and ecology of the brown dog tick Rhipicephalus sanguineus. Parasit Vectors 3(26):1–11. https://doi.org/10.1186/1756-3305-3-26
Vilela JAR, Pires MS, Da Silva CB, Peixoto MP, Falqueto A, Santo HA, Sanavria A, Massard CL, Faccini JLH (2013) Alterações clínico-hematológicas da infecção por Babesia canis vogeli em cães do município de Seropédica, Estado do Rio de Janeiro, Brasil. Rev Bras Med Vet 35(1):63–68
Sá AG, Cerqueira AMF, Odwyer LH, Macieira BM, Abreu FS, Ferreira FR, Pereira AM, Velho PB, Almosny NRP (2006) Detection and molecular characterization of Babesia canis vogeli from naturally infected Brazilian dogs. Intern J Appl Res Vet Med 4(2):163–168
Reyers F, Leisewitz AL, Lobetti RG, Milner RJ, Jacobson LS, van Zyl M (1998) Canine babesiosis in South Africa: more than one disease. Does this serve as a model for falciparum malaria? Ann Trop Med Parasitol 92(4):503–511. https://doi.org/10.1080/00034983.1998.11813308
Olicheski AT (2003) Comparação entre os métodos de coloração Panótico rápido e Giemsa para o diagnóstico de protozoários do gênero Babesia (Starcovici, 1893) e de Riquetsias do gênero Erlichia (Erlich, 1888) em cães (Canis familiares) no município de Porto Alegre, RS, Brasil. Dissertation, Universidade Federal do Rio Grande do Sul, Porto Alegre.
Ministério da Saúde (2005) Manual de Diagnóstico laboratorial de Malária. Brasília: Ministério da Saúde, pp 1–118. http://bvsms.saude.gov.br/bvs/publicacoes/malaria_diag_manual_final.pdf. Accessed 09 Sept 2019.
Shortt HE (1973) Babesia canis: the life cycle and laboratory maintenance in its arthropod and mammalian hosts. Intern J Parasitol 3:119–148. https://doi.org/10.1016/0020-7519(73)90019-2
Acknowledgements
We are grateful to the National Reference Laboratory of Riquetsioses Vectors of the Oswaldo Cruz Institute for the support in the sequencing of the samples and to the Veterinary Diagnosis Center for the donation of the samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors state that they have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
dos Santos, F.B., Gazêta , G.S., Corrêa , L.L. et al. Microscopic Detection, Hematological Evaluation and Molecular Characterization of Piroplasms from Naturally Infected Dogs in Rio de Janeiro, Brazil. Acta Parasit. 66, 1548–1560 (2021). https://doi.org/10.1007/s11686-021-00426-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-021-00426-z