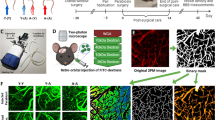
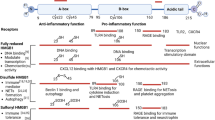
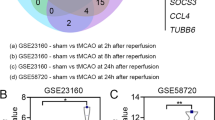
Abstract
The local microenvironment is essential to stem cell-based therapy for ischemic stroke, and spatiotemporal changes of the microenvironment in the pathological process provide vital clues for understanding the therapeutic mechanisms. However, relevant studies on microenvironmental changes were mainly confined in the acute phase of stroke, and long-term changes remain unclear. This study aimed to investigate the microenvironmental changes in the subacute and chronic phases of ischemic stroke after stem cell transplantation. Herein, induced pluripotent stem cells (iPSCs) and neural stem cells (NSCs) were transplanted into the ischemic brain established by middle cerebral artery occlusion surgery. Positron emission tomography imaging and neurological tests were applied to evaluate the metabolic and neurofunctional alterations of rats transplanted with stem cells. Quantitative proteomics was employed to investigate the protein expression profiles in iPSCs-transplanted brain in the subacute and chronic phases of stroke. Compared with NSCs-transplanted rats, significantly increased glucose metabolism and neurofunctional scores were observed in iPSCs-transplanted rats. Subsequent proteomic data of iPSCs-transplanted rats identified a total of 39 differentially expressed proteins in the subacute and chronic phases, which are involved in various ischemic stroke-related biological processes, including neuronal survival, axonal remodeling, antioxidative stress, and mitochondrial function restoration. Taken together, our study indicated that iPSCs have a positive therapeutic effect in ischemic stroke and emphasized the wide-ranging microenvironmental changes in the subacute and chronic phases.
Similar content being viewed by others
References
GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392(10159): 1859–1922
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019; 50(12): e344–e418
Stonesifer C, Corey S, Ghanekar S, Diamandis Z, Acosta SA, Borlongan CV. Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog Neurobiol 2017; 158: 94–131
Wei L, Wei ZZ, Jiang MQ, Mohamad O, Yu SP. Stem cell transplantation therapy for multifaceted therapeutic benefits after stroke. Prog Neurobiol 2017; 157: 49–78
Oki K, Tatarishvili J, Wood J, Koch P, Wattananit S, Mine Y, Monni E, Tornero D, Ahlenius H, Ladewig J, Brüstle O, Lindvall O, Kokaia Z. Human-induced pluripotent stem cells form functional neurons and improve recovery after grafting in stroke-damaged brain. Stem Cells 2012; 30(6): 1120–1133
Zhang H, Song F, Xu C, Liu H, Wang Z, Li J, Wu S, Shen Y, Chen Y, Zhu Y, Du R, Tian M. Spatiotemporal PET imaging of dynamic metabolic changes after therapeutic approaches of induced pluripotent stem cells, neuronal stem cells, and a Chinese patent medicine in stroke. J Nucl Med 2015; 56(11): 1774–1779
Andres RH, Horie N, Slikker W, Keren-Gill H, Zhan K, Sun G, Manley NC, Pereira MP, Sheikh LA, McMillan EL, Schaar BT, Svendsen CN, Bliss TM, Steinberg GK. Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain 2011; 134(6): 1777–1789
Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci 2002; 22(3): 629–634
Reis C, Wilkinson M, Reis H, Akyol O, Gospodarev V, Araujo C, Chen S, Zhang JH. A look into stem cell therapy: exploring the options for treatment of ischemic stroke. Stem Cells Int 2017; 2017: 3267352
Bacigaluppi M, Russo GL, Peruzzotti-Jametti L, Rossi S, Sandrone S, Butti E, De Ceglia R, Bergamaschi A, Motta C, Gallizioli M, Studer V, Colombo E, Farina C, Comi G, Politi LS, Muzio L, Villani C, Invernizzi RW, Hermann DM, Centonze D, Martino G. Neural stem cell transplantation induces stroke recovery by upregulating glutamate transporter GLT-1 in astrocytes. J Neurosci 2016; 36(41): 10529–10544
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126(4): 663–676
Smith DK, He M, Zhang CL, Zheng JC. The therapeutic potential of cell identity reprogramming for the treatment of aging-related neurodegenerative disorders. Prog Neurobiol 2017; 157: 212–229
Chau MJ, Deveau TC, Song M, Gu X, Chen D, Wei L. iPSC transplantation increases regeneration and functional recovery after ischemic stroke in neonatal rats. Stem Cells 2014; 32(12): 3075–3087
Sánchez-Mendoza E, Bellver-Landete V, Merino JJ, González MP, Martínez-Murillo R, Oset-Gasque MJ. Review: Could neurotransmitters influence neurogenesis and neurorepair after stroke? Neuropathol Appl Neurobiol 2013; 39(7): 722–735
Bernstock JD, Peruzzotti-Jametti L, Ye D, Gessler FA, Maric D, Vicario N, Lee YJ, Pluchino S, Hallenbeck JM. Neural stem cell transplantation in ischemic stroke: a role for preconditioning and cellular engineering. J Cereb Blood Flow Metab 2017; 37(7): 2314–2319
Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 1999; 22(9): 391–397
ElAli A, Thériault P, Rivest S. The role of pericytes in neurovascular unit remodeling in brain disorders. Int J Mol Sci 2014; 15(4): 6453–6474
Li H, You W, Li X, Shen H, Chen G. Proteomic-based approaches for the study of ischemic stroke. Transl Stroke Res 2019; 10(6): 601–606
Wen M, Jin Y, Zhang H, Sun X, Kuai Y, Tan W. Proteomic analysis of rat cerebral cortex in the subacute to long-term phases of focal cerebral ischemia-reperfusion injury. J Proteome Res 2019; 18(8): 3099–3118
Datta A, Jingru Q, Khor TH, Teo MT, Heese K, Sze SK. Quantitative neuroproteomics of an in vivo rodent model of focal cerebral ischemia/reperfusion injury reveals a temporal regulation of novel pathophysiological molecular markers. J Proteome Res 2011; 10(11): 5199–5213
Ning M, Sarracino DA, Kho AT, Guo S, Lee SR, Krastins B, Buonanno FS, Vizcaíno JA, Orchard S, McMullin D, Wang X, Lo EH. Proteomic temporal profile of human brain endothelium after oxidative stress. Stroke 2011; 42(1): 37–13
He D, Zhang Z, Lao J, Meng H, Han L, Chen F, Ye D, Zhang H, Xun Y. Proteomic analysis of the peri-infarct area after human umbilical cord mesenchymal stem cell transplantation in experimental stroke. Aging Dis 2016; 7(5): 623–634
Sung JH, Cho EH, Kim MO, Koh PO. Identification of proteins differentially expressed by melatonin treatment in cerebral ischemic injury—a proteomics approach. J Pineal Res 2009; 46(3): 300–306
Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke 1995; 26(4): 627–635
Wang J, Chao F, Han F, Zhang G, Xi Q, Li J, Jiang H, Wang J, Yu G, Tian M, Zhang H. PET demonstrates functional recovery after transplantation of induced pluripotent stem cells in a rat model of cerebral ischemic injury. J Nucl Med 2013; 54(5): 785–792
Taxin ZH, Neymotin SA, Mohan A, Lipton P, Lytton WW. Modeling molecular pathways of neuronal ischemia. Prog Mol Biol Transl Sci 2014; 123: 249–275
Yuan H, Frank JE, Hong Y, An H, Eldeniz C, Nie J, Bunevicius A, Shen D, Lin W. Spatiotemporal uptake characteristics of [18]F-2-fluoro-2-deoxy-D-glucose in a rat middle cerebral artery occlusion model. Stroke 2013; 44(8): 2292–2299
Kosi N, Alić I, Salamon I, Mitrečić D. Stroke promotes survival of nearby transplanted neural stem cells by decreasing their activation of caspase 3 while not affecting their differentiation. Neurosci Lett 2018; 666: 111–119
Zhao B, Shi QJ, Zhang ZZ, Wang SY, Wang X, Wang H. Protective effects of paeonol on subacute/chronic brain injury during cerebral ischemia in rats. Exp Ther Med 2018; 15(4): 3836–3846
Boulos S, Meloni BP, Arthur PG, Majda B, Bojarski C, Knuckey NW. Evidence that intracellular cyclophilin A and cyclophilin A/CD147 receptor-mediated ERK1/2 signalling can protect neurons against in vitro oxidative and ischemic injury. Neurobiol Dis 2007; 25(1): 54–64
Amani H, Habibey R, Shokri F, Hajmiresmail SJ, Akhavan O, Mashaghi A, Pazoki-Toroudi H. Selenium nanoparticles for targeted stroke therapy through modulation of inflammatory and metabolic signaling. Sci Rep 2019; 9(1): 6044
Garrington TP, Johnson GL. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol 1999; 11(2): 211–218
Zhu Y, Yang GY, Ahlemeyer B, Pang L, Che XM, Culmsee C, Klumpp S, Krieglstein J. Transforming growth factor-β 1 increases bad phosphorylation and protects neurons against damage. J Neurosci 2002; 22(10): 3898–3909
El-Assal ON, Besner GE. HB-EGF enhances restitution after intestinal ischemia/reperfusion via PI3K/Akt and MEK/ERK1/2 activation. Gastroenterology 2005; 129(2): 609–625
Lips DJ, Bueno OF, Wilkins BJ, Purcell NH, Kaiser RA, Lorenz JN, Voisin L, Saba-El-Leil MK, Meloche S, Pouysségur J, Pagès G, De Windt LJ, Doevendans PA, Molkentin JD. MEK1–ERK2 signaling pathway protects myocardium from ischemic injury in vivo. Circulation 2004; 109(16): 1938–1941
Astrup J, Symon L, Branston NM, Lassen NA. Cortical evoked potential and extracellular K+ and H+ at critical levels of brain ischemia. Stroke 1977; 8(1): 51–57
Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med 2008; 14(5): 497–500
Shahmoradgoli M, Mannherz O, Engel F, Heck S, Krämer A, Seiffert M, Pscherer A, Lichter P. Antiapoptotic function of charged multivesicular body protein 5: a potentially relevant gene in acute myeloid leukemia. Int J Cancer 2011; 128(12): 2865–2871
Ueno Y, Chopp M, Zhang L, Buller B, Liu Z, Lehman NL, Liu XS, Zhang Y, Roberts C, Zhang ZG. Axonal outgrowth and dendritic plasticity in the cortical peri-infarct area after experimental stroke. Stroke 2012; 43(8): 2221–2228
David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science 1981; 214(4523): 931–933
Trimarco A, Forese MG, Alfieri V, Lucente A, Brambilla P, Dina G, Pieragostino D, Sacchetta P, Urade Y, Boizet-Bonhoure B, Martinelli Boneschi F, Quattrini A, Taveggia C. Prostaglandin D2 synthase/GPR44: a signaling axis in PNS myelination. Nat Neurosci 2014; 17(12): 1682–1692
Fukuhara A, Yamada M, Fujimori K, Miyamoto Y, Kusumoto T, Nakajima H, Inui T. Lipocalin-type prostaglandin D synthase protects against oxidative stress-induced neuronal cell death. Biochem J 2012; 443(1): 75–84
Saleem S, Shah ZA, Urade Y, Doré S. Lipocalin-prostaglandin D synthase is a critical beneficial factor in transient and permanent focal cerebral ischemia. Neuroscience 2009; 160(1): 248–254
Straccia M, Carrere J, Rosser AE, Canals JM. Human t-DARPP is induced during striatal development. Neuroscience 2016; 333: 320–330
Delli Carri A, Onorati M, Lelos MJ, Castiglioni V, Faedo A, Menon R, Camnasio S, Vuono R, Spaiardi P, Talpo F, Toselli M, Martino G, Barker RA, Dunnett SB, Biella G, Cattaneo E. Developmentally coordinated extrinsic signals drive human pluripotent stem cell differentiation toward authentic DARPP-32+ medium-sized spiny neurons. Development 2013; 140(2): 301–312
Hulett JM, Walsh P, Lithgow T. Domain stealing by receptors in a protein transport complex. Mol Biol Evol 2007; 24(9): 1909–1911
Franco-Iborra S, Cuadros T, Parent A, Romero-Gimenez J, Vila M, Perier C. Defective mitochondrial protein import contributes to complex I-induced mitochondrial dysfunction and neurodegeneration in Parkinson’s disease. Cell Death Dis 2018; 9(11): 1122
Frey PA, Hegeman AD. Chemical and stereochemical actions of UDP-galactose 4-epimerase. Acc Chem Res 2013; 46(7): 1417–1426
Demirbas D, Coelho AI, Rubio-Gozalbo ME, Berry GT. Hereditary galactosemia. Metabolism 2018; 83: 188–196
Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron 2010; 67(2): 181–198
Gilgun-Sherki Y, Rosenbaum Z, Melamed E, Offen D. Antioxidant therapy in acute central nervous system injury: current state. Pharmacol Rev 2002; 54(2): 271–284
Ricciardi S, Miluzio A, Brina D, Clarke K, Bonomo M, Aiolfi R, Guidotti LG, Falciani F, Biffo S. Eukaryotic translation initiation factor 6 is a novel regulator of reactive oxygen species-dependent megakaryocyte maturation. J Thromb Haemost 2015; 13(11): 2108–2118
Kmita K, Wirth C, Warnau J, Guerrero-Castillo S, Hunte C, Hummer G, Kaila VR, Zwicker K, Brandt U, Zickermann V. Accessory NUMM (NDUFS6) subunit harbors a Zn-binding site and is essential for biogenesis of mitochondrial complex I. Proc Natl Acad Sci USA 2015; 112(18): 5685–5690
Dröse S, Stepanova A, Galkin A. Ischemic A/D transition of mitochondrial complex I and its role in ROS generation. Biochim Biophys Acta 2016; 1857(7): 946–957
Lee MO, Moon SH, Jeong HC, Yi JY, Lee TH, Shim SH, Rhee YH, Lee SH, Oh SJ, Lee MY, Han MJ, Cho YS, Chung HM, Kim KS, Cha HJ. Inhibition of pluripotent stem cell-derived teratoma formation by small molecules. Proc Natl Acad Sci USA 2013; 110(35): E3281–E3290
Ben-David U, Gan QF, Golan-Lev T, Arora P, Yanuka O, Oren YS, Leikin-Frenkel A, Graf M, Garippa R, Boehringer M, Gromo G, Benvenisty N. Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen. Cell Stem Cell 2013; 12(2): 167–179
Acknowledgements
We thank Dr. Weizhong Gu for his excellent technical assistance and expert advice on HE staining and Western blot analysis. Mass spectrometry analysis was performed in Jingjie PTM Biolab (Hangzhou) Co., Ltd. This work is sponsored by the National Key Research and Development Program of China (No. 2016YFA0100-900) and the Fund for Shanxi “1331 Project” Key Innovative Research Team.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Compliance with ethics guidelines
Yao Chen, Fahuan Song, Mengjiao Tu, Shuang Wu, Xiao He, Hao Liu, Caiyun Xu, Kai Zhang, Yuankai Zhu, Rui Zhou, Chentao Jin, Ping Wang, Hong Zhang, and Mei Tian claim that there are no conflicts of interest. All institutional and national guidelines for the care and use of laboratory animals were followed.
Supplementary Materials
Rights and permissions
About this article
Cite this article
Chen, Y., Song, F., Tu, M. et al. Quantitative proteomics revealed extensive microenvironmental changes after stem cell transplantation in ischemic stroke. Front. Med. 16, 429–441 (2022). https://doi.org/10.1007/s11684-021-0842-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11684-021-0842-9