Abstract
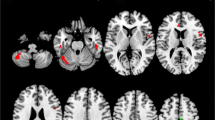
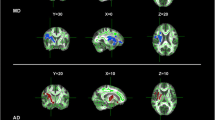
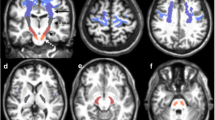
Imaging studies showed that the structure of the corpus callosum (CC) is affected in amyotrophic lateral sclerosis (ALS). Some clinical studies also suggest that interhemispheric connectivity is altered, since mirror movements seem to occur in ALS. Finally, reduced interhemispheric inhibition (IHI), studied by transcranial magnetic stimulation (TMS), has been reported. It is not known whether there is any association between these findings. Here, we studied the integrity of the CC in ALS on the morphological, the functional, the electrophysiological, and the clinical level. Twenty-seven right-handed ALS patients and 21 healthy right-handed controls were included. Mirror activity (MA) was quantified using surface EMG. Diffusion tensor imaging tractography was used to segment the CC and quantify fractional anisotropy (FA). We studied the diffusivity of the intra-axonal markers N-acetylaspartate+N-acetyl aspartyl glutamate D(tNAA) within the CC. IHI was studied as a marker of CC function using a double-pulse TMS protocol. ALS patients showed significantly decreased FA in the motor segment of the CC (p < 0.01), and IHI was significantly reduced compared to controls (p = 0.01). However, no differences were observed regarding D(tNAA) and MA. The morphological as well as the functional integrity of the CC are altered in ALS. IHI was reduced in ALS, associated with decreased FA in the motor CC. Patients did not exhibit increased MA. Also, no differences within the CC were observed using diffusion-weighted spectroscopy. IHI might serve as a marker of transcallosal pathway disruption in ALS, even before clinical deficits become apparent.



Similar content being viewed by others
References
Abrahams, S., Newton, J., Niven, E., Foley, J., & Bak, T. H. (2014). Screening for cognition and behaviour changes in ALS. Amyotrophic Lateral Sclerosis & Frontotemporal Degeneration., 15(1–2), 9–14.
Al-Chalabi, A., Hardiman, O., Kiernan, M. C., Chio, A., Rix-Brooks, B., & van den Berg, L. H. (2016). Amyotrophic lateral sclerosis: Moving towards a new classification system. The Lancet Neurology., 15(11), 1182–1194.
Andersson JLR, Jenkinson M, Smith S (2007a). Non-linear optimisation. FMRIB technical report TR07JA1 from http://www.fmrib.ox.ac.uk/analysis/techrep.
Andersson JLR, Jenkinson M, Smith S (2007b). Non-linear registration, aka Spatial normalisation FMRIB technical report TR07JA2 from http://www.fmrib.ox.ac.uk/analysis/techrep.
Bae, J. S., Simon, N. G., Menon, P., Vucic, S., & Kiernan, M. C. (2013). The puzzling case of hyperexcitability in amyotrophic lateral sclerosis. Journal of Clinical Neurology., 9(2), 65–74.
Berlucchi, G. (1990). Commisurotomy studies in animals. In F. Boller & J. Grafman (Eds.), Handbook of Neuropsychology (Vol. 4, pp. 9–47). Amsterdam: Elsevier.
Braak, H., Brettschneider, J., Ludolph, A. C., Lee, V. M., Trojanowski, J. Q., & Del Tredici, K. (2013). Amyotrophic lateral sclerosis--a model of corticofugal axonal spread. Nature reviews. Neurology, 9(12), 708–714.
Brettschneider, J., Del Tredici, K., Toledo, J. B., Robinson, J. L., Irwin, D. J., Grossman, M., et al. (2013). Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Annals of Neurology, 74(1), 20–38.
Brooks, B. R., Miller, R. G., Swash, M., & Munsat, T. L. (2000). World Federation of Neurology Research Group on motor neuron D. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic lateral sclerosis and other motor neuron disorders : official publication of the World Federation of Neurology, Research Group on Motor Neuron Diseases., 1(5), 293–299.
Chen, R., Yung, D., & Li, J. Y. (2003). Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. Journal of Neurophysiology, 89(3), 1256–1264.
Di Lazzaro, V., Oliviero, A., Pilato, F., Saturno, E., Dileone, M., Mazzone, P., et al. (2004). The physiological basis of transcranial motor cortex stimulation in conscious humans. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 115(2), 255–266.
Eisen, A., & Weber, M. (2000). Neurophysiological evaluation of cortical function in the early diagnosis of ALS. Amyotrophic Lateral Sclerosis and other Motor Neuron Disorders : Official Publication of the World Federation of Neurology, Research Group on Motor Neuron Diseases., 1(Suppl 1), S47–S51.
Ferbert, A., Priori, A., Rothwell, J. C., Day, B. L., Colebatch, J. G., & Marsden, C. D. (1992). Interhemispheric inhibition of the human motor cortex. The Journal of Physiology., 453, 525–546.
Filippini, N., Douaud, G., Mackay, C. E., Knight, S., Talbot, K., & Turner, M. R. (2010). Corpus callosum involvement is a consistent feature of amyotrophic lateral sclerosis. Neurology., 75(18), 1645–1652.
Genovese, C. R., Lazar, N. A., & Nichols, T. (2002). Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage., 15(4), 870–878.
Geraldo, A. F., Pereira, J., Nunes, P., Reimao, S., Sousa, R., Castelo-Branco, M., et al. (2018). Beyond fractional anisotropy in amyotrophic lateral sclerosis: The value of mean, axial, and radial diffusivity and its correlation with electrophysiological conductivity changes. Neuroradiology., 60(5), 505–515.
Hanajima, R., Ugawa, Y., Machii, K., Mochizuki, H., Terao, Y., Enomoto, H., Furubayashi, T., Shiio, Y., Uesugi, H., & Kanazawa, I. (2001). Interhemispheric facilitation of the hand motor area in humans. The Journal of Physiology., 531(Pt 3), 849–859.
Harris-Love, M. L., Perez, M. A., Chen, R., & Cohen, L. G. (2007). Interhemispheric inhibition in distal and proximal arm representations in the primary motor cortex. Journal of Neurophysiology, 97(3), 2511–2515.
Hofer, S., & Frahm, J. (2006). Topography of the human corpus callosum revisited--comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. NeuroImage., 32(3), 989–994.
Hubers, A., Orekhov, Y., & Ziemann, U. (2008). Interhemispheric motor inhibition: Its role in controlling electromyographic mirror activity. The European Journal of Neuroscience., 28(2), 364–371.
Karandreas, N., Papadopoulou, M., Kokotis, P., Papapostolou, A., Tsivgoulis, G., & Zambelis, T. (2007). Impaired interhemispheric inhibition in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis : Official Publication of the World Federation of Neurology Research Group on Motor Neuron Diseases., 8(2), 112–118.
Lule, D., Burkhardt, C., Abdulla, S., Bohm, S., Kollewe, K., Uttner, I., et al. (2015). The Edinburgh cognitive and Behavioural amyotrophic lateral sclerosis screen: A cross-sectional comparison of established screening tools in a German-Swiss population. Amyotrophic Lateral Sclerosis & Frontotemporal Degeneration, 16(1–2), 16–23.
Mayston, M. J., Harrison, L. M., & Stephens, J. A. (1999). A neurophysiological study of mirror movements in adults and children. Annals of Neurology, 45(5), 583–594.
Muller, H. P., Unrath, A., Ludolph, A. C., & Kassubek, J. (2007). Preservation of diffusion tensor properties during spatial normalization by use of tensor imaging and fibre tracking on a normal brain database. Physics in Medicine and Biology, 52(6), N99–N109.
Muller, H. P., Lule, D., Unrath, A., Ludolph, A. C., Riecker, A., & Kassubek, J. (2011). Complementary image analysis of diffusion tensor imaging and 3-dimensional t1-weighted imaging: White matter analysis in amyotrophic lateral sclerosis. Journal of Neuroimaging : Official Journal of the American Society of Neuroimaging., 21(1), 24–33.
Muller, H. P., Unrath, A., Huppertz, H. J., Ludolph, A. C., & Kassubek, J. (2012). Neuroanatomical patterns of cerebral white matter involvement in different motor neuron diseases as studied by diffusion tensor imaging analysis. Amyotrophic lateral sclerosis : official publication of the World Federation of Neurology Research Group on Motor Neuron Diseases., 13(3), 254–264.
Nicolay, K., Braun, K. P., Graaf, R. A., Dijkhuizen, R. M., & Kruiskamp, M. J. (2001). Diffusion NMR spectroscopy. NMR in Biomedicine, 14(2), 94–111.
Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia., 9(1), 97–113.
Palombo, M., Shemesh, N., Ronen, I., & Valette, J. (2018). Insights into brain microstructure from in vivo DW-MRS. NeuroImage., 182, 97–116.
Perez, M. A., Wise, S. P., Willingham, D. T., & Cohen, L. G. (2007). Neurophysiological mechanisms involved in transfer of procedural knowledge. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience., 27(5), 1045–1053.
Reischauer, C., Gutzeit, A., Neuwirth, C., Fuchs, A., Sartoretti-Schefer, S., Weber, M., & Czell, D. (2018). In-vivo evaluation of neuronal and glial changes in amyotrophic lateral sclerosis with diffusion tensor spectroscopy. NeuroImage Clinical., 20, 993–1000.
Ronen, I., Ercan, E., & Webb, A. (2013). Axonal and glial microstructural information obtained with diffusion-weighted magnetic resonance spectroscopy at 7T. Frontiers in Integrative Neuroscience, 7, 13.
Rossini, P. M., Berardelli, A., Deuschl, G., & Hallett, M. (1999). Maertens de Noordhout AM, Paulus W, et al. Applications of Magnetic Cortical Stimulation. The International Federation of Clinical Neurophysiology. Electroencephalography and Clinical Neurophysiology Supplement., 52, 171–185.
Rueckert, D., Sonoda, L. I., Hayes, C., Hill, D. L., Leach, M. O., & Hawkes, D. J. (1999). Nonrigid registration using free-form deformations: Application to breast MR images. IEEE Transactions on Medical Imaging, 18(8), 712–721.
Salerno, A., & Georgesco, M. (1998). Double magnetic stimulation of the motor cortex in amyotrophic lateral sclerosis. Electroencephalography and Clinical Neurophysiology, 107(2), 133–139.
Smith, S. M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17(3), 143–155.
Smith, S. M., Jenkinson, M., Johansen-Berg, H., Rueckert, D., Nichols, T. E., Mackay, C. E., Watkins, K. E., Ciccarelli, O., Cader, M. Z., Matthews, P. M., & Behrens, T. E. (2006). Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage., 31(4), 1487–1505.
Unrath, A., Muller, H. P., Riecker, A., Ludolph, A. C., Sperfeld, A. D., & Kassubek, J. (2010). Whole brain-based analysis of regional white matter tract alterations in rare motor neuron diseases by diffusion tensor imaging. Human Brain Mapping, 31(11), 1727–1740.
Upadhyay, J., Hallock, K., Erb, K., Kim, D. S., & Ronen, I. (2007). Diffusion properties of NAA in human corpus callosum as studied with diffusion tensor spectroscopy. Magnetic Resonance in Medicine, 58(5), 1045–1053.
Verstraete, E., Turner, M. R., Grosskreutz, J., Filippi, M., & Benatar, M. (2015). Attendees of the 4th Ni Sm. Mind the gap: The mismatch between clinical and imaging metrics in ALS. Amyotrophic Lateral Sclerosis & Frontotemporal Degeneration., 16(7–8), 524–529.
Vucic, S., Cheah, B. C., & Kiernan, M. C. (2009). Defining the mechanisms that underlie cortical hyperexcitability in amyotrophic lateral sclerosis. Experimental Neurology, 220(1), 177–182.
Wahl, M., Hübers, A., Lauterbach-Soon, B., Hattingen, E., Jung, P., Cohen, L. G., & Ziemann, U. (2011). Motor callosal disconnection in early relapsing-remitting multiple sclerosis. Human Brain Mapping, 32(6), 846–855.
Wittstock, M., Wolters, A., & Benecke, R. (2007). Transcallosal inhibition in amyotrophic lateral sclerosis. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 118(2), 301–307.
Wittstock, M., Meister, S., Walter, U., Benecke, R., & Wolters, A. (2011). Mirror movements in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis : Official Publication of the World Federation of Neurology Research Group on Motor Neuron Diseases., 12(6), 393–397.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest related to this study.
Ethical approval
All procedures performed were in accordance with the ethical standards of the institutional research commitee of the University of Ulm and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hübers, A., Böckler, B., Abaei, A. et al. Functional and structural impairment of transcallosal motor fibres in ALS: a study using transcranial magnetic stimulation, diffusion tensor imaging, and diffusion weighted spectroscopy. Brain Imaging and Behavior 15, 748–757 (2021). https://doi.org/10.1007/s11682-020-00282-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-020-00282-x