Abstract
Purpose
The aim of this study was to evaluate the integrity of the corticospinal tracts (CST) in patients with SCA3 and age- and gender-matched healthy control subjects using diffusion tensor imaging (DTI). We also looked at the clinical correlates of such diffusivity abnormalities.
Methods
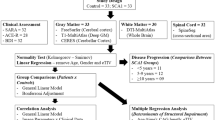
We assessed 2 cohorts from different Brazilian centers: cohort 1 (n = 29) scanned in a 1.5 T magnet and cohort 2 (n = 91) scanned in a 3.0 T magnet. We used Pearson’s coefficients to assess the correlation of CST DTI parameters and ataxia severity (expressed by SARA scores).
Results
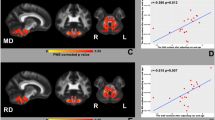
Two different results were obtained. Cohort 1 showed no significant between-group differences in DTI parameters. Cohort 2 showed significant between-group differences in the FA values in the bilateral precentral gyri (p < 0.001), bilateral superior corona radiata (p < 0.001), bilateral posterior limb of the internal capsule (p < 0.001), bilateral cerebral peduncle (p < 0.001), and bilateral basis pontis (p < 0.001). There was moderate correlation between CST diffusivity parameters and SARA scores in cohort 2 (Pearson correlation coefficient: 0.40–0.59).
Conclusion
DTI particularly at 3 T is able to uncover and quantify CST damage in SCA3. Moreover, CST microstructural damage may contribute with ataxia severity in the disease.


Similar content being viewed by others
References
Kawaguchi Y, Okamoto T, Taniwaki M, Aizawa M, Inoue M, Katayama S, Kawakami H, Nakamura S, Nishimura M, Akiguchi I, Kimura J, Narumiya S, Kakizuka A (1994) CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nat Genet 8:221–228. https://doi.org/10.1038/ng1194-221
Nakano K, Spence A, Dawson D (1972) Machado disease. Neurology 22:49–55. https://doi.org/10.1212/WNL.22.1.49
Rosenberg RN, Fowler HL, de Magalhães J et al (1977) Azorean disease of the nervous system. N Engl J Med 297:729–730. https://doi.org/10.1056/NEJM197709292971318
Dürr A, Stevanin G, Cancel G et al (1996) Spinocerebellar ataxia 3 and Machado-Joseph disease: clinical, molecular, and neuropathological features. Ann Neurol 39:490–499. https://doi.org/10.1002/ana.410390411
Pedroso JL, França MC, Braga-Neto P et al (2013) Nonmotor and extracerebellar features in Machado-Joseph disease: a review. Mov Disord 28:1200–1208. https://doi.org/10.1002/mds.25513
Riess O, Rüb U, Pastore A, Bauer P, Schöls L (2008) SCA3: neurological features, pathogenesis and animal models. Cerebellum 7:125–137. https://doi.org/10.1007/s12311-008-0013-4
Rüb U, Brunt ER, Deller T (2008) New insights into the pathoanatomy of spinocerebellar ataxia type 3 (Machado-Joseph disease). Curr Opin Neurol 21:111–116. https://doi.org/10.1097/WCO.0b013e3282f7673d
de Rezende TJR, D’Abreu A, Guimarães RP et al (2015) Cerebral cortex involvement in Machado-Joseph disease. Eur J Neurol 22:277–283. https://doi.org/10.1111/ene.12559
Murata Y, Yamaguchi S, Kawakami H, Imon Y, Maruyama H, Sakai T, Kazuta T, Ohtake T, Nishimura M, Saida T, Chiba S, Oh-i T, Nakamura S (1998) Characteristic magnetic resonance imaging findings in Machado-Joseph disease. Arch Neurol 55:33–37. https://doi.org/10.1001/archneur.55.1.33
Onodera O, Idezuka J, Igarashi S, Takiyama Y, Endo K, Takano H, Oyake M, Tanaka H, Inuzuka T, Hayashi T, Yuasa T, Ito J, Miyatake T, Tsuji S (1998) Progressive atrophy of cerebellum and brainstem as a function of age and the size of the expanded CAG repeats in the MJD1 gene in Machado-Joseph disease. Ann Neurol 43:288–296. https://doi.org/10.1002/ana.410430305
Peng H, Liang X, Long Z, Chen Z, Shi Y, Xia K, Meng L, Tang B, Qiu R, Jiang H (2019) Gene-related cerebellar neurodegeneration in SCA3/MJD: a case-controlled imaging-genetic study. Front Neurol 10:1–10. https://doi.org/10.3389/fneur.2019.01025
Farrar MA, Vucic S, Nicholson G, Kiernan MC (2016) Motor cortical dysfunction develops in spinocerebellar ataxia type 3. Clin Neurophysiol 127:3418–3424. https://doi.org/10.1016/j.clinph.2016.09.005
Jhunjhunwala K, Prashanth DK, Netravathi M, Jain S, Purushottam M, Pal PK (2013) Alterations in cortical excitability and central motor conduction time in spinocerebellar ataxias 1, 2 and 3: a comparative study. Parkinsonism Relat Disord 19:306–311. https://doi.org/10.1016/j.parkreldis.2012.11.002
Wu X, Liao X, Zhan Y, Cheng C, Shen W, Huang M, Zhou Z, Wang Z, Qiu Z, Xing W, Liao W, Tang B, Shen L (2017) Microstructural alterations in asymptomatic and symptomatic patients with spinocerebellar ataxia type 3: a tract-based spatial statistics study. Front Neurol 8:1–9. https://doi.org/10.3389/fneur.2017.00714
Rezende TJR, de Paiva JLR, Martinez ARM, Lopes-Cendes I, Pedroso JL, Barsottini OGP, Cendes F, França MC Jr (2018) Structural signature of SCA3: from presymptomatic to late disease stages. Ann Neurol 84:401–408. https://doi.org/10.1002/ana.25297
Schmitz-Hübsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, Giunti P, Globas C, Infante J, Kang JS, Kremer B, Mariotti C, Melegh B, Pandolfo M, Rakowicz M, Ribai P, Rola R, Schöls L, Szymanski S, van de Warrenburg B, Dürr A, Klockgether T, Fancellu R (2006) Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology 66:1717–1720. https://doi.org/10.1212/01.wnl.0000219042.60538.92
Zhuang J, Hrabe J, Kangarlu A, Xu D, Bansal R, Branch CA, Peterson BS (2006) Correction of eddy-current distortions in diffusion tensor images using the known directions and strengths of diffusion gradients. J Magn Reson Imaging 24:1188–1193. https://doi.org/10.1002/jmri.20727
Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC (1998) Automated image registration: I. General methods and intrasubject, intramodality validation. J Comput Assist Tomogr 22:139–152. https://doi.org/10.1097/00004728-199801000-00027
Tang X, Yoshida S, Hsu J, Huisman TAGM, Faria AV, Oishi K, Kutten K, Poretti A, Li Y, Miller MI, Mori S (2014) Multi-contrast multi-atlas parcellation of diffusion tensor imaging of the human brain. PLoS One 9:e96985. https://doi.org/10.1371/journal.pone.0096985
Towns J, Cockerill T, Dahan M, Foster I, Gaither K, Grimshaw A, Hazlewood V, Lathrop S, Lifka D, Peterson GD, Roskies R, Scott JR, Wilkins-Diehr N (2014) XSEDE: Accelerating scientific discovery. Comput Sci Eng 16:62–74. https://doi.org/10.1109/MCSE.2014.80
Qin W, Yu CS, Zhang F et al (2009) Effects of echo time on diffusion quantification of brain white matter at 1.5 T and 3.0 T. Magn Reson Med 61:755–760. https://doi.org/10.1002/mrm.21920
Chou MC, Kao EF, Mori S (2013) Effects of b-value and echo time on magnetic resonance diffusion tensor imaging-derived parameters at 1.5 t: a voxel-wise study. J Med Biol Eng 33:45–50. https://doi.org/10.5405/jmbe.1126
Chung AW, Thomas DL, Ordidge RJ, Clark CA (2013) Diffusion tensor parameters and principal eigenvector coherence: Relation to b-value intervals and field strength. Magn Reson Imaging 31:742–747. https://doi.org/10.1016/j.mri.2012.11.014
Paulson HL, Das SS, Crino PB, Perez MK, Patel SC, Gotsdiner D, Fischbeck KH, Pittman RN (1997) Machado-Joseph disease gene product is a cytoplasmic protein widely expressed in brain. Ann Neurol 41:453–462. https://doi.org/10.1002/ana.410410408
Wu Y, Peng Y, Wang Y (2014) An insight into advances in the pathogenesis and therapeutic strategies of spinocerebellar ataxia type 3. Rev Neurosci 26:95–104. https://doi.org/10.1515/revneuro-2014-0040
D’Abreu A, França M, Appenzeller S et al (2009) Axonal dysfunction in the deep white matter in Machado-Joseph disease. J Neuroimaging 19:9–12. https://doi.org/10.1111/j.1552-6569.2008.00260.x
Jardim LB, Pereira ML, Silveira I, Ferro A, Sequeiros J, Giugliani R (2001) Neurologic findings in Machado-Joseph disease. Arch Neurol 58:899. https://doi.org/10.1001/archneur.58.6.899
Pulido-Valdeolivas I, Gómez-Andrés D, Sanz-Gallego I, Rausell E, Arpa J (2016) Patterns of motor signs in spinocerebellar ataxia type 3 at the start of follow-up in a reference unit. Cerebellum Ataxias 3:1–10. https://doi.org/10.1186/s40673-016-0042-6
Guang WY, Du J, Ling WJ et al (2009) Six cases of SCA3/MJD patients that mimic hereditary spastic paraplegia in clinic. J Neurol Sci 285:121–124. https://doi.org/10.1016/j.jns.2009.06.027
Song Y, Liu Y, Zhang N, Long L (2015) Spinocerebellar ataxia type 3/machado-joseph disease manifested as spastic paraplegia: a clinical and genetic study. Exp Ther Med 9:417–420. https://doi.org/10.3892/etm.2014.2136
Song SK, Yoshino J, Le TQ et al (2005) Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 26:132–140. https://doi.org/10.1016/j.neuroimage.2005.01.028
Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH (2003) Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 20:1714–1722. https://doi.org/10.1016/j.neuroimage.2003.07.005
Sun SW, Liang HF, Trinkaus K, Cross AH, Armstrong RC, Song SK (2006) Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magn Reson Med 55:302–308. https://doi.org/10.1002/mrm.20774
Karlsborg M, Rosenbaum S, Wiegell MR, Simonsen H, Larsson HBW, Werdelin LM, Gredal O (2004) Corticospinal tract degeneration and possible pathogenesis in ALS evaluated by MR diffusion tensor imaging. Amyotroph Lateral Scler Other Mot Neuron Disord 5:136–140. https://doi.org/10.1080/14660820410018982
Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, Khan J, Polak MA, Glass JD (2004) Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol 185:232–240. https://doi.org/10.1016/j.expneurol.2003.10.004
Yabe I, Matsushima M, Soma H, Basri R, Sasaki H (2008) Usefulness of the scale for assessment and rating of ataxia (SARA). J Neurol Sci 266:164–166. https://doi.org/10.1016/j.jns.2007.09.021
Bürk K, Sival DA (2018) Scales for the clinical evaluation of cerebellar disorders. Handb Clin Neurol 154:329–339. https://doi.org/10.1016/B978-0-444-63956-1.00020-5
Schmitz-Hübsch T, Fimmers R, Rakowicz M et al (2010) Responsiveness of different rating instruments in spinocerebellar ataxia patients. Neurology 74:678–684. https://doi.org/10.1212/WNL.0b013e3181d1a6c9
Paap BK, Roeske S, Durr A, Schöls L, Ashizawa T, Boesch S, Bunn LM, Delatycki MB, Giunti P, Lehéricy S, Mariotti C, Melegh J, Pandolfo M, Tallaksen CME, Timmann D, Tsuji S, Schulz JB, van de Warrenburg BP, Klockgether T (2016) Standardized assessment of hereditary ataxia patients in clinical studies. Mov Disord Clin Pract 3:230–240. https://doi.org/10.1002/mdc3.12315
Kang JS, Klein JC, Baudrexel S, Deichmann R, Nolte D, Hilker R (2014) White matter damage is related to ataxia severity in SCA3. J Neurol 261:291–299. https://doi.org/10.1007/s00415-013-7186-6
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Inada, B.S.Y., Rezende, T.J.R., Pereira, F.V. et al. Corticospinal tract involvement in spinocerebellar ataxia type 3: a diffusion tensor imaging study. Neuroradiology 63, 217–224 (2021). https://doi.org/10.1007/s00234-020-02528-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-020-02528-3