Abstract
Men and women show important differences in clinical conditions in which deficits in cognitive control are implicated. We used functional magnetic resonance imaging to examine gender differences in the neural processes of cognitive control during a stop-signal task. We observed greater activation in men, compared to women, in a wide array of cortical and sub-cortical areas, during stop success (SS) as compared to stop error (SE). Conversely, women showed greater regional brain activation during SE > SS, compared to men. Furthermore, compared to women, men engaged the right inferior parietal lobule to a greater extent during post-SE go compared to post-go go trials. Women engaged greater posterior cingulate cortical activation than men during post-SS slowing in go trial reaction time (RT) but did not differ during post-SE slowing in go trial RT. These findings extended our previous results of gender differences in regional brain activation during response inhibition. The results may have clinical implications by, for instance, helping initiate studies to understand why women are more vulnerable to depression while men are more vulnerable to impulse control disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A psychological process of interest to both basic and clinical neuroscientists is cognitive control. As a hallmark of higher cortical function, cognitive control allows flexible behaviors and adaptations to changing environment. Altered cognitive control has been implicated in a number of clinical conditions, including substance dependence (de Wit and Richards 2004; Baler and Volkow 2006; Fellows 2004; Goldstein and Volkow 2002; Jentsch and Taylor 1999; Kalenscher et al. 2006; Moeller et al. 2001) and depression (Compton et al. 2008; Fales et al. 2008; Hardin et al. 2007; Harvey et al. 2005; Holmes and Pizzagalli 2008a, b; Matthews et al. 2008; see also Discussion). Men and women show important clinical differences in these illnesses (Brienza and Stein 2002; Brady and Randall 1999; Hyde et al. 2008; Leach et al. 2008; Sinha and Rounsaville 2002).
Gender differences in brain activity during cognitive processing have begun to receive attention in the recent literature of functional neuroimaging. For instance, gender differences in regional brain activation have been observed in behavioral tasks requiring word generation (Bell et al. 2006; Gizewski et al. 2006), visual word learning (Chen et al. 2007), spatial attention (Bell et al. 2006), execution of a visuospatial plan (Boghi et al. 2006; Unterrainer et al. 2005), working memory (Bell et al. 2006; Gaab et al. 2003; Goldstein et al. 2005; Mitchell 2007; Ragland et al. 2000; Speck et al. 2000; Schweinsburg et al. 2005), and target and novelty detection (Gur et al. 2007). Although many of these findings are new and require replication, gender differences in functional brain organization seem to be obtained from basic sensory and motor processing to complex cognitive and emotive functions (Hamann and Canli 2004; Li et al. 2006a; Wager et al. 2003; Wager and Ochsner 2005; see also Cosgrove et al. 2007 for a review of sex differences in brain structure and chemistry).
To date, however, we know very little about the neural mechanisms underlying the gender differences in cognitive control. In particular, whether and how men and women differ in the component processes of cognitive control have not been investigated. The present study aims to explore this important gap of knowledge. The stop signal task (SST) is one of the most common paradigms used to examine cognitive control (Logan 1994; Logan and Cowan 1984). In the SST, the dominant or more frequent stimulus constitutes a go signal requiring subjects to respond within a time window therefore setting up a prepotent response tendency. The other, less frequent, no-go or stop signal instructs subjects to refrain from making the response. With a procedure to track participants’ performance, the difficulty of the task can be adjusted trial by trial such that participants make errors half of the time despite their constant effort to avoid making errors. Using this paradigm, distinct component processes, including response inhibition, error detection and post-error behavioral adjustment are evoked (Chevrier et al. 2007; Li et al. 2006a, 2008c, d; Li and Sinha 2008; Liotti et al. 2005; Schachar et al. 2004; Stahl and Gibbons 2007; Stevens et al. 2009). Prior neuroimaging studies have attempted to elucidate the neural mechanisms underlying each of the component processes of cognitive control during the SST (Chevrier et al. 2007; Li et al. 2006c, 2008a, c; Stevens et al. 2009).
The current work extends our previous preliminary study examining gender differences in motor response inhibition during the SST (Li et al. 2006a), specifically recruiting more men and women subjects in order to examine gender differences in the component processes of cognitive control. We briefly speculate on the implications of the current findings for the gender differences in depressive and impulse control disorders.
Methods
Subjects and behavioral task
Sixty adult healthy subjects (30 females, 22–42 years of age, all right-handed and using their right hand to respond) were included in this study. The current sample includes 40 subjects who were recruited in our previous work (Li et al. 2006a). All subjects signed a written consent after details of the study were explained, in accordance to institute guidelines and procedures approved by the Yale Human Investigation Committee.
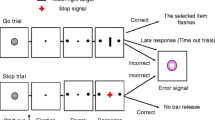
We employed a simple reaction time (RT) task in this stop-signal paradigm (Fig. 1). There were two trial types: “go” and “stop,” randomly intermixed. A small dot appeared on the screen to engage attention and eye fixation at the beginning of a go trial. After a randomized time interval (fore-period) anywhere between 1 and 5 s, the dot turned into a circle, prompting the subjects to quickly press a button. The circle vanished at button press or after 1 s had elapsed, whichever came first, and the trial terminated. A premature button press prior to the appearance of the circle also terminated the trial. Three quarters of all trials were go trials. In a stop trial, an additional “X,” the “stop” signal, appeared after the go signal. The subjects were told to withhold button press upon seeing the stop signal. Likewise, a trial terminated at button press or when 1 s had elapsed since the appearance of the stop signal. The stop trials constituted the remaining one quarter of the trials. There was an inter-trial-interval of 2 s.
Stop signal paradigm. In “go” trials (75%) observers responded to the go signal (a circle) and in “stop” trials (25%) they had to withhold the response when they saw the stop signal (an X). In both trials the go signal appeared after a randomized time interval between 1 to 5 s (the fore-period or FP, uniform distribution) following the appearance of the fixation point. The go signal disappeared at the time of button press or when 1 s had elapsed, whichever came first, ending the trial. In a stop trial, the stop signal replaced the go signal by a time delay—the stop signal delay (SSD). The SSD was updated according to a staircase procedure, whereby it increased and decreased by 64 ms following a stop success and stop error trial, respectively
It would be easier for the subject to withhold the response if the stop signal appeared immediately or early after the go signal, and the reverse applied if the time interval between the stop and the go signals (or the stop-signal delay, SSD) was extended. The SSD started at 200 ms and varied from one stop trial to the next according to a staircase procedure: if the subject succeeded in withholding the response, the SSD increased by 64 ms, making it more difficult to succeed again in the next stop trial; conversely, if a subject failed, SSD decreased by 64 ms, making it easier for the next stop trial. With the staircase procedure, a “critical” SSD could be computed that represents the time delay required for the subject to succeed in withholding a response half of the time in the stop trials (Levitt 1970).
One way to understand the stop signal task (SST) is in terms of a horse race model with a go process and a stop process racing toward a finishing line (Logan 1994). The go process prepares and generates the movement while the stop process inhibits movement initiation: whichever process finishes first determines whether a response will be initiated or not. Importantly, the go and stop processes race toward the activation threshold independently. Thus, the time required for the stop signal to be processed so a response is withheld (i.e., stop signal reaction time or SSRT) can be computed on the basis of the go trial RT distribution and the odds of successful inhibitions for different time delays between go and stop signals. This is done by estimating the critical SSD at which a response can be correctly stopped in approximately 50% of the stop trials. With the assumptions of this “horse-race” model, the SSRT could then be computed in the current tracking stop signal task for each individual subject by subtracting the critical SSD from the median go trial RT. Generally speaking, the SSRT is the time required for a subject to cancel the movement after seeing the stop signal. A longer SSRT indicates poor response inhibition.
Subjects were instructed to respond to the go signal quickly while keeping in mind that a stop signal could come up in a small number of trials. Prior to the fMRI study each subject had a practice session outside the scanner. Each subject completed four 10-min runs of the task with the SSD updated automatically by the program within individual runs and by the investigator across runs. Depending on the actual stimulus timing (e.g., trials varied in fore-period duration) and speed of response, the total number of trials varied slightly across subjects in an experiment. There were approximately 105 trials in a 10-min run, including approximately 79 go trials and 26 stop trials. With the staircase procedure we anticipated that the subjects would succeed in withholding their response in approximately 50% of the stop trials.
We computed the fore-period effect as an index of motor preparedness during the SST (Li et al. 2005, 2006b, c; Tseng and Li 2008). Briefly, longer fore-period is associated with faster response time (Bertelson and Tisseyre 1968; Woodrow 1914). Thus, RT of go trials with a fore-period between 3 and 5 s were compared to those with one between 1 and 3 s, and the effect size of RT difference was defined as fore-period effect. We also computed a behavioral index of error monitoring. It is known that in a reaction time (RT) task the RT of a correct response is prolonged following an error, compared to other correct responses, and this prolonged RT is thought to reflect cognitive processes involved in error monitoring (Li et al. 2006b; Rabbit 1966). We thus computed the RT difference between the go trials that followed a stop error and those that followed another go trial, and termed this RT difference “post-error slowing” (Hajcak et al. 2003; Li et al. 2006b, 2008a).
Imaging protocol
Conventional T1-weighted spin echo sagittal anatomical images were acquired for slice localization using a 3T scanner (Siemens Trio). Anatomical images of the functional slice locations were next obtained with spin echo imaging in the axial plane parallel to the AC-PC line with TR = 300 ms, TE = 2.5 ms, bandwidth = 300 Hz/pixel, flip angle = 60°, field of view = 220 × 220 mm, matrix = 256 × 256, 32 slices with slice thickness = 4 mm and no gap. Functional, blood oxygen level dependent (BOLD) signals were then acquired with a single-shot gradient echo echoplanar imaging (EPI) sequence. Thirty-two axial slices parallel to the AC-PC line covering the whole brain were acquired with TR = 2,000 ms, TE = 25 ms, bandwidth = 2,004 Hz/pixel, flip angle = 85°, field of view = 220 × 220 mm, matrix = 64 × 64, 32 slices with slice thickness = 4 mm and no gap. Three hundred images were acquired in each run for a total of four runs.
Data analysis: spatial pre-processing of brain images
Data were analyzed with Statistical Parametric Mapping version 2 (Wellcome Department of Imaging Neuroscience, University College London, U.K.). Images from the first five TRs at the beginning of each trial were discarded to enable the signal to achieve steady-state equilibrium between RF pulsing and relaxation. Images of each individual subject were first corrected for slice timing and realigned (motion-corrected). A mean functional image volume was constructed for each subject for each run from the realigned image volumes. These mean images were normalized to an MNI (Montreal Neurological Institute) EPI template with affine registration followed by nonlinear transformation (Ashburner and Friston 1999; Friston et al. 1995a). The normalization parameters determined for the mean functional volume were then applied to the corresponding functional image volumes for each subject. Finally, images were smoothed with a Gaussian kernel of 10 mm at Full Width at Half Maximum. The data were high-pass filtered (1/128 Hz cutoff) to remove low-frequency signal drifts.
Data analysis: statistical modeling
We constructed two general linear models (GLM) to examine processes involved in response inhibition, error detection (first GLM), post-error processing, and post-stop trial behavioral adjustment (second GLM). Events of interest were distinguished and modeled for each individual subject, using the GLM with the onsets of go signal in each event of interest convolved with a canonical hemodynamic response function (HRF) and with the temporal derivative of the canonical HRF and entered as regressors in the model (Friston et al. 1995b). Realignment parameters in all six dimensions were also entered in the model. Serial autocorrelation was corrected by a first-degree autoregressive or AR(1) model. The general linear model estimated the component of variance that could be explained by each of the regressors. In the first-level analysis, we constructed for each individual subject a number of different statistical contrasts. The con or contrast (difference in β) images of the first-level analysis were then used for the second-level group statistics (random effect analysis, Penny and Holmes 2007).
In the first GLM, four types of trial outcomes were distinguished: go success (G), go error (F), stop success (SS) and stop error (SE). Because each G trial was associated with a different reaction time (RT), we entered a column of G trial onset parametrically modulated by its corresponding RT as a regressor in the model. Likewise, because SS and SE trials came with different stop-signal delays (SSD), we entered columns of SS and SE trial onsets parametrically modulated by SSD in the model. We contrasted SS > SE trials to evaluate brain regions that were differentially activated during stop success and error, which could reflect (stop) signal monitoring, in addition to response inhibition (Li et al. 2006c, 2008d). Thus, as in our previous study, we performed a median split separately for the 30 men and women subjects based on their SSRT and performed a 2 (men vs. women) by 2 (short vs. long SSRT) ANOVA (Li et al. 2006c). The two SSRT groups of subjects (men or women) did not differ in any other aspects of stop signal performance including stop success rate and were thus indistinguishable in the extent of signal monitoring. We applied the reverse contrast SE > SS to evaluate regional brain activation during error processing including affective responses that might differ between stop success and error trials. Compared to stop success, stop error also involved motor activity. We thus expected to observe greater activity in motor structures during SE > SS as evidence validating the behavioral paradigm and statistical construct.
In the second GLM, G, F, SS, and SE trials were first distinguished. G trials were divided into those that followed a G (pG), SS (pSS) and SE (pSE) trial (Li et al. 2008c). Furthermore, pSS and pSE trials were divided into those that increased in RT (pSSi and pSEi, respectively) and those that did not increase in RT (pSSni and pSEni), to allow the isolation of neural processes involved in post-conflict/error behavioral adjustment (Li et al. 2008a). To determine whether a pSS/pSE trial increased or did not increase in RT, it was compared to the pG trials that preceded it in time during each session. The pG trials that followed the pSS or pSE trial were not included for comparison because the neural/cognitive processes associated with these pG trials occurred subsequent to and thus could not have a causal effect on the pSS or pSE trial (Li et al. 2008a). These regressors were used to develop a second GLM. We constructed for each individual subject the following statistical contrasts: SS vs. SE (to compare with the first GLM and verify the model); pSS vs. pG; pSE vs. pG; pSS vs. pSE (to evaluate post-error processes); and pSSi vs. pSSni, and pSEi vs. pSEni (to identify activations associated with post-conflict and post-error adjustment in RT, respectively).
Brain regions were identified using an atlas (Duvernoy 2003; Mai et al. 2003). In Region of interest (ROI) analysis, we used MarsBaR (Brett et al. 2002; http://marsbar.sourceforge.net/) to derive for each individual subject the effect size of activity change for the ROIs. The functional ROIs were defined based on activated clusters from whole brain analysis. All voxel activations are presented in MNI coordinates.
Results
Behavioral performance during the SST
Men and women did not differ in their general task performance, including the success rates of both go and stop trials, median go trial reaction time (RT), stop signal reaction time (SSRT), post-error slowing (PES), and fore-period (FP) effect (Table 1). Equitable stop signal performance allowed us to exclude motivation, vigilance, or other general psychological factors in the attribution of gender differences. Behavioral results are also listed separately for SSRT grouping. Within men or women, individuals with short and long SSRT did not differ in any other aspects of stop signal performance (0.136<Ps<0.848, two-tailed 2-sample t tests).
Brain imaging results
Because of the multiple contrasts examined in the current study, our plan was first to test for gender differences for each specific contrast using analysis of variance (ANOVA) or two-sided 2-sample t test, at a threshold of p < 0.001, uncorrected, and five voxels in the extent of activation (see below). For each contrast that showed significant gender differences, we further examined the regional brain activations associated with that contrast separately for women and men, in order to identify the sources of variation.
The results showed that men and women did not differ in the neural correlates of response inhibition in the contrast of short vs. long SSRT (ANOVA). By comparing men and women directly for the contrast SS>SE, we showed that men activated a number of cortical and subcortical structures during SS, as compared to SE, more than women. These regions included the frontopolar and perigenual anterior cingulate cortices, and superior colliculus (Fig. 2; Table 2). We derived the effect sizes of the contrast SS > SE for each of the seven regions that showed activity difference between men and women (Fig. 3). For instance, both men and women showed greater activation (but to a different extent) in the superior colliculus during SE compared to SS. On the other hand, perigenual anterior cingulate cortex (pgACC) showed greater activity during SE, in contrast to SS, in women, while the reverse seems to be the case in men. Figure 4 shows the regional brain activation for SS vs. SE trials, separately for men and women. Overall, men seemed to show greater activation during SS>SE compared to women in frontal brain regions, while women appeared to show greater activation during SE>SS in cingulate brain regions as well as many subcortical structures such as the thalamus.
Brain regions showing greater activation during stop success (SS) > stop error (SE) in men, as compared to women. BOLD contrasts are superimposed on a T1 structural image in sagittal sections from x = −16 to x = +4. Adjacent sections are 4 mm apart. Color bar represents voxel T value. Activated regions are summarized in Table 2. Apart from the pre-supplementary motor area, these brain regions are distinct from the structures that were implicated in response inhibition in our previous work (shown here in BLUE, Li et al. 2006c), suggesting that error processing may play a primary role in mediating these differences
Effect sizes for the contrast stop success (SS) > stop error (SE) plotted separately for men and women for the seven regions in Table 2. Vertical bars represented the standard deviation. Men and women showed opposite pattern of activity between SS and SE for the pre-supplementary motor area (a), perigenual anterior cingulate cortex (d), and cerebellum (f). Men and women both showed greater activation during SS compared to SE in superior (c, e) and orbital (g) frontal gyri. Men and women both showed less activation during SS compared to SE in the superior colliculus (b)
Brain regions showing differential activation between stop success (SS) and stop error (SE) in women (a) and men (b). BOLD contrasts are superimposed on a T1 structural image in axial sections from y = −42 to y = +66. Adjacent sections are 6 mm apart. Color bar represents voxel T values: warm color: SS>SE; winter color: SE>SS. Image orientation is neurological (i.e., Right=Right). Square boxes highlight the approximate locations of the seven regions that differ in the contrast between men and women (see also Table 2)
We examined whether men and women differ in regional brain activity after they encountered a stop success or error trial. Thus, we contrasted between post-SS go (pSS) and post-go go (pG) trials, between post-SE go (pSE) and pG trials, and between pSS and pSE trials. Only the contrast between pSE and pG trials revealed significant gender differences. Compared to women, men showed greater activity in the right inferior parietal cortex (angular gyrus), superior frontal and inferior temporal gyri during pSE compared to pG go trials (Table 3). Men showed greater activity in all of the four structures during pSE compared to the pG trials, while the reverse was true of women. Examining men and women separately, we observed that men and women both showed less activation of the paracentral lobules, posterior cingulate cortex and precuneus, and anterior cingulate cortex, during pSE, compared to pG, trials (Fig. 5). Men also showed greater activation in the right frontal and parietal cortices during pSE compared to pG trials.
Brain regions showing differential activation between post-stop error go (pSE) and post-go go (pG) trials in women (a) and men (b). BOLD contrasts are superimposed on a T1 structural image in axial sections from y = −42 to y = +66. Adjacent sections are 6 mm apart. Color bar represents voxel T values: warm color: pSE>pG; winter color: pSE<pG. Image orientation is neurological (i.e., Right=Right). Overall, men and women both showed cerebral “deactivation” following an error. Unlike women, however, men also activated a few brain regions following an error. Square boxes highlight the approximate locations of the four regions that differ in the contrast between men and women
We examined the neural processes involved in post-SS and post-SE behavioral adjustment (Li et al. 2008a). The results showed that women but not men activated the posterior cingulate cortex (PCC) during post-SS slowing (x = 16, y = −52, z = 40; voxel Z = 3.20, Fig. 6). In contrast, both men and women activated and did not differ in right ventrolateral prefrontal cortex (VLPFC) during post-SE slowing in go trial RT (Fig. 6). Consistent with our earlier work, VLPFC activity was not correlated with PES (p’s > 0.6, Pearson regression, for men and women separately or for all subjects combined). PCC activity was not correlated with post-SS slowing in women (p > 0.6, Pearson regression).
Brain regions showing increased activity during post-stop success slowing in go trial RT (pSSi>pSSni) for women (a) and men (b), and during post-stop error slowing in go trial RT (pSEi>pSEni) for women (c) and men (d). BOLD contrasts are superimposed on a T1 structural image. Color bar represents voxel T values. Image orientation is neurological (i.e., Right=Right). Both women and men showed activation in the ventrolateral prefrontal cortex (VLPFC) during post-error slowing. Women but not men also showed activation of the posterior cingulate cortex during post-stop success slowing
Discussion
Women and men did not differ in terms of their behavioral performance in the stop signal task. This finding was important as it allowed us to examine performance-invariant gender differences in cognitive control as embodied by the stop signal task.
Response inhibition indexed by the stop signal reaction time (SSRT)
At a threshold of p < 0.001, uncorrected, women and men did not differ in regional brain activation during response inhibition as indicated by the SSRT. Although men and women each showed greater activation of the pre-SMA and the caudate tail, when short and long SSRT groups were contrasted (data not shown, replicating Li et al. 2006a), the differences did not manifest with direct group comparison.
Error processing
Compared with women, men showed greater activation in cerebellum, frontal cortical areas, and the superior colliculus, for the contrast stop success (SS) > stop error (SE). These differences in regional activation largely reflect greater cerebral and cerebellar responses to SE, compared to SS, in women, and greater responses to SS, compared to SE, in men. For instance, thalamus including the superior colliculus activated during SE, compared to SS, both in men and women, but this differential activation was more prominent in women. These differential responses can be more clearly observed by viewing Fig. 3, where the effect sizes of SS > SE trials are plotted for individual ROIs. Greater thalamic activation during SE perhaps reflected more extensive performance monitoring and feedback or saliency processing in women, compared to men (Christoffels et al. 2007; Hester et al. 2004; Maltby et al. 2005; Ogawa et al. 2006; Rubia et al. 2007).
Men showed greater activity in the perigenual anterior cingulate cortex (ACC), anterior medial superior frontal cortex (SFC), and cerebellum during SS > SE, while women showed the opposite pattern of responses. The anterior medial SFC and cerebellum has been implicated in learning and updating “cognitive sets” during performance of behavioral tasks that require executive functions (Beauchamp et al. 2003; Collette et al. 2007; Dreher et al. 2002). In particular, cerebellum has long been implicated in feedback processing during motor control (Christensen et al. 2007; Diedrichsen et al. 2005; Grafton et al. 2008; Jenmalm et al. 2006; Milner et al. 2007; Ogawa et al. 2006). Recently Ito speculated about a role of the cerebellum in embodying an internal model of mental activities other than movement control (Ito 2008). These opposite patterns of cerebral responses between SS and SE trials perhaps suggested that there were fundamental differences in the way men and women perform the stop signal task.
Post-error processing
Both men and women showed less activation of many areas in the default circuitry during go trials following a stop error, as compared to those following a go trial (Fig. 5). This pattern of BOLD signal “deactivation” could suggest that participants were “geared up” for better task performance after they committed a mistake (Greicius et al. 2003; Li et al. 2008c, d; Raichle et al. 2001; Shulman et al. 1997; Tomasi et al. 2006; Weissman et al. 2006). Interestingly, while this pattern of “deactivation” predominated both in men and women, men showed greater activation in a number of frontal cortical structures as well as in cerebellum. A direct comparison revealed greater activation in men of the right angular gyrus, bilateral superior frontal gyri, and right inferior temporal gyrus during post-SE go compared to post-go go trials, while the reverse was true of women. Intraparietal and superior frontal cortices are involved in top-down, goal-directed selection of stimuli and responses (see Corbetta and Shulman 2002, for a review). This gender difference thus suggests that, in addition to “deactivating” the default mode brain regions, men also engaged an endogenous attention system in preparation for an upcoming trial, compared to women. This finding along with greater error-related activity in women, as compared to men, may indicate fundamental differences in the temporal dynamics with which men and women respond to errors during a cognitive task that requires moment-to-moment monitoring of performance.
Post-stop trial behavioral adjustment
Women and men both involved the right ventrolateral prefrontal cortices (VLPFC) during post-error behavioral adjustment. However, compared to men, who did not show specific regional brain activity, women activated the posterior cingulate cortex during post-SS slowing in go trial RT. Our previous fMRI study of 40 healthy (20 men) subjects showed VLPFC activity during post-SE slowing (PES) in go trial RT (Li et al. 2008a). In addition, we were not able to identify a specific area to mediate post-SS slowing and suggested that post-SS slowing might not involve an active decision process as engaged during PES. The observation in the current study of greater posterior cingulate activity indicates that, compared to men, women engaged the posterior cingulate cortex (PCC) during post-SS slowing, as PCC neurons were implicated in signaling behavioral outcomes and action orientations (McCoy and Platt 2005). On the other hand, this PCC activity may also reflect that, compared to men, women were involved to a greater extent in “mental reflection” during pSS slowing (Vogt and Laureys 2005).
Potential implications for gender differences in psychopathology
Men and women are noted to have different clinical profiles for psychiatric conditions. For instance, compared to women, men are more likely to have diagnosis of substance abuse, which, directly or indirectly, reflect their differences in cognitive control (Berkowitz and Perkins 1987; Huselid and Cooper 1992; Nagoshi et al. 1991; Thomas 1995; Rosenblitt et al. 2001; Whiteside and Lynam 2003; Windle 1990). The interplay between stressful life events and cognitive style may explain at least, in part, the greater prevalence of major depression in women than in men (Mazure and Maciejewski 2003). Cognitive vulnerability has been suggested as an important mediator of gender differences in the clinical profiles of bipolar disorder (Alloy et al. 2006). Cognitive behavioral therapies have shown effectiveness in the management of irritable bowel syndrome, a condition that predominantly affects women (Heitkemper et al. 2004). Studies have also suggested an important role of cognitive distortion of body schema in the pathogenesis of eating disorders, which affect predominantly women (Powell and Hendricks 1999). Characterizing the brain processes—the intermediate phenotypes—of cognitive control thus represents an important step in understanding the gender differences found in these disorders.
Prior neuroimaging studies revealed a number of structures that link depression to deficits in cognitive control (Compton et al. 2008; Fales et al. 2008; Hardin et al. 2007; Harvey et al. 2005; Holmes and Pizzagalli 2008a, b; Matthews et al. 2008). However, gender differences have not been explored in these studies. The current findings may thus provide useful information to investigate the neural basis of gender differences in depression. For instance, Staley and colleagues showed that serotonin transporter availability decreased in the diencephalon in patients with major depression, compared to healthy controls, and such a decrease was more prominent in women than men (Staley et al. 2006). Given the role of serotonergic neurotransmission in cognitive control and impulsivity (Gorwood et al. 2000; Mann et al. 2001; Purselle and Nemeroff 2003; Retz et al. 2004; Strobel et al. 2007; Walderhaug et al. 2007; see, however, Clark et al. 2005 for negative evidence), the current findings of greater error-evoked thalamic activity in women seem to indicate a potentially useful intermediate neural phenotype to further elucidate gender differences in depression.
On another speculative note, although the differences do not manifest in direct group comparison, men engage pre-supplementary motor area and caudate nucleus involved in motor response inhibition (Li et al. 2006a) while women activate the caudate tail mediating visual discrimination learning (Brown et al. 1995; Li et al. 2006a; Seger 2008) when individuals with short and long stop signal reaction time are contrasted. This finding suggests that prepotent motor responses—a “habit”—are more easily evoked in men, a tendency that could predispose them to impulse control including substance use disorders. Studies are warranted to investigate whether the differential engagement of response inhibition and association learning circuits could serve as a neural signature of behavioral impulsivity.
Conclusions
In summary, we showed that men and women differ in the neural processes underlying cognitive control. In particular, women showed greater error-related activity, compared to men. Since behavioral errors frequently engage cortical and subcortical activity that can also be evoked during affective processing, these results suggest the importance in further pursuing gender differences in the effects of affective processing on cognitive control (Pessoa 2008).
References
Alloy, L. B., Abramson, L. Y., Walshaw, P. D., Keyser, J., & Gerstein, R. K. (2006). A cognitive vulnerability-stress perspective on bipolar spectrum disorders in a normative adolescent brain, cognitive, and emotional development context. Development and Psychopathology, 18, 1055–1103. doi:10.1017/S0954579406060524.
Ashburner, J., & Friston, K. J. (1999). Nonlinear spatial normalization using basis functions. Human Brain Mapping, 7, 254–266. doi:10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G.
Baler, R. D., & Volkow, N. D. (2006). Drug addiction: the neurobiology of disrupted self-control. Trends in Molecular Medicine, 12, 559–566. doi:10.1016/j.molmed.2006.10.005.
Beauchamp, M. H., Dagher, A., Aston, J. A., & Doyon, J. (2003). Dynamic functional changes associated with cognitive skill learning of an adapted version of the Tower of London task. NeuroImage, 20, 1649–1660. doi:10.1016/j.neuroimage.2003.07.003.
Bell, E. C., Willson, M. C., Wilman, A. H., Dave, S., & Silverstone, P. H. (2006). Males and females differ in brain activation during cognitive tasks. NeuroImage, 30, 529–538. doi:10.1016/j.neuroimage.2005.09.049.
Berkowitz, A. D., & Perkins, H. W. (1987). Recent research on gender differences in collegiate alcohol use. Journal of American College Health, 36, 123–129.
Bertelson, P., & Tisseyre, F. (1968). The time-course of preparation with regular and irregular foreperiods. Quarterly Journal of Experimental Psychology, 20, 297–300. doi:10.1080/14640746808400165.
Boghi, A., Rasetti, R., Avidano, F., Manzone, C., Orsi, L., D’Agata, F., et al. (2006). The effect of gender on planning: An fMRI study using the Tower of London task. NeuroImage, 33, 999–1010. doi:10.1016/j.neuroimage.2006.07.022.
Brady, K. T., & Randall, C. L. (1999). Gender differences in substance use disorders. Psychiatric Clinics of North America, 22, 241–252. doi:10.1016/S0193-953X(05)70074-5.
Brett, M., Anton, J.-L., Valabregue, R., & Poline, J.-P. (2002). Region of interest analysis using an SPM toolbox. Abstract presented at the 8th International Conference on Functional Mapping of the Human Brain, June 2–6, Sendai, Japan.
Brienza, R. S., & Stein, M. D. (2002). Alcohol use disorders in primary care: do gender-specific differences exist? Journal of General Internal Medicine, 17, 387–397.
Brown, V. J., Desimone, R., & Mishkin, M. (1995). Responses of cells in the tail of the caudate nucleus during visual discrimination learning. Journal of Neurophysiology, 74, 1083–1094.
Chen, C., Xue, G., Dong, Q., Jin, Z., Li, T., Xue, F., et al. (2007). Sex determines the neurofunctional predictors of visual word learning. Neuropsychologia, 45, 741–747. doi:10.1016/j.neuropsychologia.2006.08.018.
Chevrier, A. D., Noseworthy, M. D., & Schachar, R. (2007). Dissociation of response inhibition and performance monitoring in the stop signal task using event-related fMRI. Human Brain Mapping, 28, 1347–1358. doi:10.1002/hbm.20355.
Christensen, M. S., Lundbye-Jensen, J., Petersen, N., Geertsen, S. S., Paulson, O. B., & Nielsen, J. B. (2007). Watching your foot move—an fMRI study of visuomotor interactions during foot movement. Cerebral Cortex (New York, N.Y.), 17, 1906–1917. doi:10.1093/cercor/bhl101.
Clark, L., Roiser, J. P., Cools, R., Rubinsztein, D. C., Sahakian, B. J., & Robbins, T. W. (2005). Stop signal response inhibition is not modulated by tryptophan depletion or the serotonin transporter polymorphism in healthy volunteers: implications for the 5-HT theory of impulsivity. Psychopharmacology, 182, 570–578. doi:10.1007/s00213-005-0104-6.
Collette, F., Van der Linden, M., Laureys, S., Arigoni, F., Delfiore, G., Degueldre, C., et al. (2007). Mapping the updating process: common and specific brain activations across different versions of the running span task. Cortex, 43, 146–158. doi:10.1016/S0010-9452(08)70452-0.
Compton, R. J., Lin, M., Vargas, G., Carp, J., Fineman, S. L., & Quandt, L. C. (2008). Error detection and posterror behavior in depressed undergraduates. Emotion (Washington, D.C.), 8, 58–67. doi:10.1037/1528-3542.8.1.58.
Corbetta, M., & Shulman, G. L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews. Neuroscience, 3, 201–215. doi:10.1038/nrn755.
Cosgrove, K. P., Mazure, C. M., & Staley, J. K. (2007). Evolving knowledge of sex differences in brain structure, function, and chemistry. Biological Psychiatry, 62, 847–855. doi:10.1016/j.biopsych.2007.03.001.
de Wit, H., & Richards, J. B. (2004). Dual determinants of drug use in humans: reward and impulsivity. Nebraska Symposium on Motivation, 50, 19–55.
Diedrichsen, J., Hashambhoy, Y., Rane, T., & Shadmehr, R. (2005). Neural correlates of reach errors. Journal of Neuroscience, 25, 9919–9931. doi:10.1523/JNEUROSCI.1874-05.2005.
Dreher, J. C., Koechlin, E., Ali, S. O., & Grafman, J. (2002). The roles of timing and task order during task switching. NeuroImage, 17, 95–109. doi:10.1006/nimg.2002.1169.
Duvernoy, H. M. (2003). The human brain, 2nd Edn. Austria: Springer-Verlag, Wien.
Fales, C. L., Barch, D. M., Rundle, M. M., Mintun, M. A., Snyder, A. Z., Cohen, J. D., et al. (2008). Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biological Psychiatry, 63, 377–384. doi:10.1016/j.biopsych.2007.06.012.
Fellows, L. K. (2004). The cognitive neuroscience of human decision making: a review and conceptual framework. Behavioral and Cognitive Neuroscience Reviews, 3, 159–172. doi:10.1177/1534582304273251.
Friston, K. J., Ashburner, J., Frith, C. D., Polone, J.-B., Heather, J. D., & Frackowiak, R. S. J. (1995a). Spatial registration and normalization of images. Human Brain Mapping, 2, 165–189. doi:10.1002/hbm.460030303.
Friston, K. J., Holmes, A. P., Worsley, K. J., Poline, J.-B., Frith, C. D., & Frackowiak, R. S. J. (1995b). Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping, 2, 189–210. doi:10.1002/hbm.460020402.
Gaab, N., Keenan, J. P., & Schlaug, G. (2003). The effects of gender on the neural substrates of pitch memory. Journal of Cognitive Neuroscience, 15, 810–820. doi:10.1162/089892903322370735.
Gizewski, E. R., Krause, E., Wanke, I., Forsting, M., & Senf, W. (2006). Gender-specific cerebral activation during cognitive tasks using functional MRI: comparison of women in mid-luteal phase and men. Neuroradiology, 48, 14–20.
Goldstein, R. Z., & Volkow, N. D. (2002). Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry, 159, 1642–1652. doi:10.1176/appi.ajp.159.10.1642.
Goldstein, J. M., Jerram, M., Poldrack, R., Anagnoson, R., Breiter, H. C., Makris, N., et al. (2005). Sex differences in prefrontal cortical brain activity during fMRI of auditory verbal working memory. Neuropsychology, 19, 509–519. doi:10.1037/0894-4105.19.4.509.
Gorwood, P., Batel, P., Adès, J., Hamon, M., & Boni, C. (2000). Serotonin transporter gene polymorphisms, alcoholism, and suicidal behavior. Biological Psychiatry, 48, 259–264. doi:10.1016/S0006-3223(00)00840-4.
Grafton, S. T., Schmitt, P., Van Horn, J., & Diedrichsen, J. (2008). Neural substrates of visuomotor learning based on improved feedback control and prediction. NeuroImage, 39, 1383–1395. doi:10.1016/j.neuroimage.2007.09.062.
Greicius, M. D., Krasnow, B., Reiss, A. L., & Menon, V. (2003). Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America, 100, 253–258. doi:10.1073/pnas.0135058100.
Gur, R. C., Turetsky, B. I., Loughead, J., Waxman, J., Snyder, W., Ragland, J. D., et al. (2007). Hemodynamic responses in neural circuitries for detection of visual target and novelty: an event-related fMRI study. Human Brain Mapping, 28, 263–274. doi:10.1002/hbm.20319.
Hajcak, G., McDonald, N., & Simons, R. F. (2003). To err is autonomic: error-related brain potentials, ANS activity, and post-error compensatory behavior. Psychophysiology, 40, 895–903. doi:10.1111/1469-8986.00107.
Hamann, S., & Canli, T. (2004). Individual differences in emotion processing. Current Opinion in Neurobiology, 14, 233–238. doi:10.1016/j.conb.2004.03.010.
Hardin, M. G., Schroth, E., Pine, D. S., & Ernst, M. (2007). Incentive-related modulation of cognitive control in healthy, anxious, and depressed adolescents: development and psychopathology related differences. Journal of Child Psychology and Psychiatry and Allied Disciplines, 48, 446–454. doi:10.1111/j.1469-7610.2006.01722.x.
Harvey, P. O., Fossati, P., Pochon, J. B., Levy, R., Lebastard, G., Lehéricy, S., et al. (2005). Cognitive control and brain resources in major depression: an fMRI study using the n-back task. NeuroImage, 26, 860–869. doi:10.1016/j.neuroimage.2005.02.048.
Heitkemper, M., Jarrett, M., & Bond, E. F. (2004). Irritable bowel syndrome in women: a common health problem. Nursing Clinics of North America, 39, 69–81. doi:10.1016/j.cnur.2003.11.016.
Hester, R., Fassbender, C., & Garavan, H. (2004). Individual differences in error processing: a review and reanalysis of three event-related fMRI studies using the GO/NOGO task. Cerebral Cortex (New York, N.Y.), 14, 986–994. doi:10.1093/cercor/bhh059.
Holmes, A. J., & Pizzagalli, D. A. (2008a). Spatiotemporal dynamics of error processing dysfunctions in major depressive disorder. Archives of General Psychiatry, 65, 179–188. doi:10.1001/archgenpsychiatry.2007.19.
Holmes, A. J., & Pizzagalli, D. A. (2008b). Response conflict and frontocingulate dysfunction in unmedicated participants with major depression. Neuropsychologia, 46, 2904–2913.
Huselid, R. F., & Cooper, M. L. (1992). Gender roles as mediators of sex differences in adolescent alcohol use and abuse. Journal of Health and Social Behavior, 33, 348–362. doi:10.2307/2137313.
Hyde, J. S., Mezulis, A. H., & Abramson, L. Y. (2008). The ABCs of depression: integrating affective, biological, and cognitive models to explain the emergence of the gender difference in depression. Psychological Review, 115, 291–313. doi:10.1037/0033-295X.115.2.291.
Ito, M. (2008). Control of mental activities by internal models in the cerebellum. Nature Reviews. Neuroscience, 9, 304–313. doi:10.1038/nrn2332.
Jenmalm, P., Schmitz, C., Forssberg, H., & Ehrsson, H. H. (2006). Lighter or heavier than predicted: neural correlates of corrective mechanisms during erroneously programmed lifts. Journal of Neuroscience, 26, 9015–9021. doi:10.1523/JNEUROSCI.5045-05.2006.
Jentsch, J. D., & Taylor, J. R. (1999). Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl), 146, 373–390. doi:10.1007/PL00005483.
Kalenscher, T., Ohmann, T., & Güntürkün, O. (2006). The neuroscience of impulsive and self-controlled decisions. International Journal of Psychophysiology, 62, 203–211. doi:10.1016/j.ijpsycho.2006.05.010.
Leach, L. S., Christensen, H., Mackinnon, A. J., Windsor, T. D., & Butterworth, P. (2008). Gender differences in depression and anxiety across the adult lifespan: the role of psychosocial mediators. Social Psychiatry and Psychiatric Epidemiology, 43, 983–998.
Levitt, H. (1970). Transformed up-down methods in psychoacoustics. Journal of the Acoustical Society of America, 49, 467–477. doi:10.1121/1.1912375.
Li, C.-S. R., & Sinha, R. (2008). Frontolimbic dysfunctions and substance abuse: neuroimaging evidence and a cognitive-analytic perspective. Neuroscience and Biobehavioral Reviews, 32, 581–597. doi:10.1016/j.neubiorev.2007.10.003.
Li, C.-S. R., Mathalon, D. H., & Krystal, J. H. (2005). Fore-period effect and stop signal processing time. Experimental Brain Research, 167, 305–309. doi:10.1007/s00221-005-0110-2.
Li, C.-S. R., Huang, C., Constable, R. T., & Sinha, R. (2006a). Gender differences in the neural correlates of response inhibition during a stop signal task. NeuroImage, 32, 1918–1929. doi:10.1016/j.neuroimage.2006.05.017.
Li, C.-S. R., Milivojevic, V., Kemp, K. A., Hong, K., & Sinha, R. (2006b). Performance monitoring and stop signal inhibition in abstinent patients with cocaine dependence. Drug and Alcohol Dependence, 149, 129–138.
Li, C.-S. R., Huang, C., Constable, T., & Sinha, R. (2006c). Imaging response inhibition in a stop signal task—neural correlates independent of signal monitoring and post-response processing. Journal of Neuroscience, 26, 186–192. doi:10.1523/JNEUROSCI.3741-05.2006.
Li, C.-S. R., Huang, C., Yan, P., Paliwal, P., Constable, R. T., & Sinha, R. (2008a). Neural correlates of post-error slowing in a stop signal task. Journal of Cognitive Neuroscience, 20, 1021–1029. doi:10.1162/jocn.2008.20071.
Li, C.-S. R., Huang, C., Yan, P., Bhagawar, Z., Milivojevic, V., & Sinha, R. (2008b). Neural correlates of impulse control during stop signal inhibition in cocaine dependent men. Neuropsychopharmacology, 33, 1798–1806. doi:10.1038/sj.npp. 1301568.
Li, C.-S. R., Yan, P., Chao, H. H.-A., Sinha, R., Paliwal, P., Constable, R. T., et al. (2008c). Error-specific medial cortical and subcortical activity during the stop signal task—a functional magnetic resonance imaging study. Neuroscience, 155, 1142–1151. doi:10.1016/j.neuroscience.2008.06.062.
Li, C.-S. R., Yan, P., Sinha, R., & Lee, T. W. (2008d). The subcortical processes of motor response inhibition during a stop signal task. NeuroImage, 41, 1352–1363. doi:10.1016/j.neuroimage.2008.01.068.
Liotti, M., Pliszka, S. R., Perez, R., Kothmann, D., & Woldorff, M. G. (2005). Abnormal brain activity related to performance monitoring and error detection in children with ADHD. Cortex, 41, 377–388. doi:10.1016/S0010-9452(08)70274-0.
Logan, G. D. (1994). Inhibitory processes. In D. Dagenbach & T. H. Carr (Eds.), Attention, memory and language, pp. 189–239. San Diego: Academic.
Logan, G. D., & Cowan, W. B. (1984). On the ability to inhibit thought and action: a theory of an act of control. Psychological Review, 91, 295–327. doi:10.1037/0033-295X.91.3.295.
Mai, J. K., Paxinos, G., & Asheuer, J. K. (2003). Atlas of the human brain (2nd ed.). New York, NY: Academic.
Maltby, N., Tolin, D. F., Worhunsky, P., O’Keefe, T. M., & Kiehl, K. A. (2005). Dysfunctional action monitoring hyperactivates frontal-striatal circuits in obsessive–compulsive disorder: an event-related fMRI study. NeuroImage, 24, 495–503. doi:10.1016/j.neuroimage.2004.08.041.
Mann, J. J., Brent, D. A., & Arango, V. (2001). The neurobiology and genetics of suicide and attempted suicide: a focus on the serotonergic system. Neuropsychopharmacology, 24, 467–477. doi:10.1016/S0893-133X(00)00228-1.
Matthews, S. C., Strigo, I. A., Simmons, A. N., Yang, T. T., & Paulus, M. P. (2008). Decreased functional coupling of the amygdala and supragenual cingulate is related to increased depression in unmedicated individuals with current major depressive disorder. Journal of Affective Disorders, 111, 13–20.
Mazure, C. M., & Maciejewski, P. K. (2003). The interplay of stress, gender and cognitive style in depressive onset. Archives of Women’s Mental Health, 6, 5–8. doi:10.1007/s00737-002-0161-3.
McCoy, A. N., & Platt, M. L. (2005). Expectations and outcomes: decision-making in the primate brain. Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology, 191, 201–211.
Milner, T. E., Franklin, D. W., Imamizu, H., & Kawato, M. (2007). Central control of grasp: manipulation of objects with complex and simple dynamics. NeuroImage, 36, 388–395. doi:10.1016/j.neuroimage.2007.01.057.
Mitchell, R. L. (2007). fMRI delineation of working memory for emotional prosody in the brain: commonalities with the lexico-semantic emotion network. NeuroImage, 36, 1015–1025.
Moeller, F. G., Barratt, E. S., Dougherty, D. M., Schmitz, J. M., & Swann, A. C. (2001). Psychiatric aspects of impulsivity. American Journal of Psychiatry, 158, 1783–1793. doi:10.1176/appi.ajp.158.11.1783.
Nagoshi, C. T., Wilson, J. R., & Rodriguez, L. A. (1991). Impulsivity, sensation seeking, and behavioral and emotional responses to alcohol. Alcoholism, Clinical and Experimental Research, 15, 661–667. doi:10.1111/j.1530-0277.1991.tb00575.x.
Ogawa, K., Inui, T., & Sugio, T. (2006). Separating brain regions involved in internally guided and visual feedback control of moving effectors: an event-related fMRI study. NeuroImage, 32, 1760–1770. doi:10.1016/j.neuroimage.2006.05.012.
Penny, W., & Holmes, A. J. (2007). Random effect analysis. In K. J. Friston, J. T. Ashburner, S. J. Kiebel, T. E. Nichols, & W. D. Penny (Eds.), Statistical parametric mapping. Burlington, MA: Academic Press.
Pessoa, L. (2008). On the relationship between emotion and cognition. Nature Reviews. Neuroscience, 9, 148–158. doi:10.1038/nrn2317.
Powell, M. R., & Hendricks, B. (1999). Body schema, gender, and other correlates in nonclinical populations. Genetic, Social, and General Psychology Monographs, 125, 333–412.
Purselle, D. C., & Nemeroff, C. B. (2003). Serotonin transporter: a potential substrate in the biology of suicide. Neuropsychopharmacology, 28, 613–619. doi:10.1038/sj.npp.1300092.
Rabbit, P. M. A. (1966). Errors and error correction in choice-response tasks. Journal of Experimental Psychology, 71, 264–272. doi:10.1037/h0022853.
Ragland, J. D., Coleman, A. R., Gur, R. C., Glahn, D. C., & Gur, R. E. (2000). Sex differences in brain-behavior relationships between verbal episodic memory and resting regional cerebral blood flow. Neuropsychologia, 38, 451–461. doi:10.1016/S0028-3932(99)00086-X.
Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A., & Shulman, G. L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98, 676–682. doi:10.1073/pnas.98.2.676.
Retz, W., Retz-Junginger, P., Supprian, T., Thome, J., & Rösler, M. (2004). Association of serotonin transporter promoter gene polymorphism with violence: relation with personality disorders, impulsivity, and childhood ADHD psychopathology. Behavioral Sciences & the Law, 22, 415–425. doi:10.1002/bsl.589.
Rosenblitt, J. C., Soler, H., Johnson, S. E., & Quadagno, D. M. (2001). Sensation seeking and hormones in men and women: exploring the link. Hormones and Behavior, 40, 396–402. doi:10.1006/hbeh.2001.1704.
Rubia, K., Smith, A. B., Taylor, E., & Brammer, M. (2007). Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Human Brain Mapping, 28, 1163–1177. doi:10.1002/hbm.20347.
Schachar, R. J., Chen, S., Logan, G. D., Ornstein, T. J., Crosbie, J., Ickowicz, A., et al. (2004). Evidence for an error monitoring deficit in attention deficit hyperactivity disorder. Journal of Abnormal Child Psychology, 32, 285–293. doi:10.1023/B:JACP.0000026142.11217.f2.
Schweinsburg, A. D., Nagel, B. J., & Tapert, S. F. (2005). fMRI reveals alteration of spatial working memory networks across adolescence. Journal of the International Neuropsychological Society, 11, 631–644. doi:10.1017/S1355617705050757.
Seger, C. A. (2008). How do the basal ganglia contribute to categorization? Their roles in generalization, response selection, and learning via feedback. Neuroscience and Biobehavioral Reviews, 32, 265–278. doi:10.1016/j.neubiorev.2007.07.010.
Shulman, G. L., Fiez, J. A., Corbetta, M., Buckner, R. I., Miezin, F. M., Raichle, M. E., et al. (1997). Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. Journal of Cognitive Neuroscience, 9, 648–663. doi:10.1162/jocn.1997.9.5.648.
Sinha, R., & Rounsaville, B. J. (2002). Sex differences in depressed substance abusers. Journal of Clinical Psychiatry, 63, 616–627.
Speck, O., Ernst, T., Braun, J., Koch, C., Miller, E., & Chang, L. (2000). Gender differences in the functional organization of the brain for working memory. NeuroReport, 11, 2581–2585. doi:10.1097/00001756-200008030-00046.
Stahl, J., & Gibbons, H. (2007). Dynamics of response-conflict monitoring and individual differences in response control and behavioral control: an electrophysiological investigation using a stop-signal task. Clinical Neurophysiology, 118, 581–596. doi:10.1016/j.clinph.2006.10.023.
Staley, J. K., Sanacora, G., Tamagnan, G., Maciejewski, P. K., Malison, R. T., Berman, R. M., et al. (2006). Sex differences in diencephalon serotonin transporter availability in major depression. Biological Psychiatry, 59, 40–47. doi:10.1016/j.biopsych.2005.06.012.
Stevens, M. C., Kiehl, K. A., Pearlson, G. D., & Calhoun, V. D. (2009). Brain network dynamics during error commission. Human Brain Mapping, 30(1), 24–37.
Strobel, A., Dreisbach, G., Müller, J., Goschke, T., Brocke, B., & Lesch, K. P. (2007). Genetic variation of serotonin function and cognitive control. Journal of Cognitive Neuroscience, 19, 1923–1931. doi:10.1162/jocn.2007.19.12.1923.
Tomasi, D., Ernst, T., Caparelli, E. C., & Chang, L. (2006). Common deactivation patterns during working memory and visual attention tasks: an intra-subject fMRI study at 4 Tesla. Human Brain Mapping, 27, 694–705. doi:10.1002/hbm.20211.
Tseng, Y. C., & Li, C.-S. R. (2008). The effects of response readiness and error monitoring on saccade countermanding. The Open Psychology Journal, 1, 18–25. doi:10.2174/1874350100801010018.
Unterrainer, J. M., Ruff, C. C., Rahm, B., Kaller, C. P., Spreer, J., Schwarzwald, R., et al. (2005). The influence of sex differences and individual task performance on brain activation during planning. NeuroImage, 24, 586–590. doi:10.1016/j.neuroimage.2004.09.020.
Vogt, B. A., & Laureys, S. (2005). Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Progress in Brain Research, 150, 205–217. doi:10.1016/S0079-6123(05)50015-3.
Wager, T. D., & Ochsner, K. N. (2005). Sex differences in the emotional brain. NeuroReport, 16, 85–87. doi:10.1097/00001756-200502080-00001.
Wager, T. D., Phan, K. L., Liberzon, I., & Taylor, S. F. (2003). Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. NeuroImage, 19, 513–531. doi:10.1016/S1053-8119(03)00078-8.
Walderhaug, E., Magnusson, A., Neumeister, A., Lappalainen, J., Lunde, H., Refsum, H., et al. (2007). Interactive effects of sex and 5-HTTLPR on mood and impulsivity during tryptophan depletion in healthy people. Biological Psychiatry, 62, 593–599. doi:10.1016/j.biopsych.2007.02.012.
Weissman, D. H., Roberts, K. C., Visscher, K. M., & Woldorff, M. G. (2006). The neural bases of momentary lapses in attention. Nature Neuroscience, 9, 971–978. doi:10.1038/nn1727.
Whiteside, S. P., & Lynam, D. R. (2003). Understanding the role of impulsivity and externalizing psychopathology in alcohol abuse: application of the UPPS impulsive behavior scale. Experimental and Clinical Psychopharmacology, 11, 210–217. doi:10.1037/1064-1297.11.3.210.
Windle, M. (1990). A longitudinal study of antisocial behaviors in early adolescence as predictors of late adolescent substance use: gender and ethnic group differences. Journal of Abnormal Psychology, 99, 86–91. doi:10.1037/0021-843X.99.1.86.
Woodrow, H. (1914). The measurement of attention. Psychological Monographs, 17, 1–158.
Acknowledgements
This study was supported by the BIRWCH K12 Award, funded by the NIH Office of Research on Women’s Health (Mazure), a Clinician Scientist K12 award (Rounsaville), NIH grants R03DA022395 (Li), R01DA023248 (Li), and P50-DA16556 (Sinha). It was also supported by a research grant from the Alcoholic Beverage Medical Research Foundation (Li) and the Clinical Translational Science Award (NIH-UL1 RR024139) awarded to Yale University (Sherwin).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Appendix: A list of abbreviations
Appendix: A list of abbreviations
AC-PC: anterior commissure- posterior commissure; dACC: dorsal anterior cingulate cortex; rACC: rostral anterior cingulate cortex; BA: Brodmann area; BOLD: blood oxygenation level dependent; dACC: dorsal anterior cingulate cortex; EPI: echo-planar imaging; F: go error trials; FDR: false discovery rate; fMRI: functional magnetic resonance imaging; FP: fore-period; FWE: family-wise error; G: go success trials; GLM: generalized linear model; HRF: hemodynamic response function; IFC: inferior frontal cortex; MNI: Montreal Neurological Institute; PES: post-error slowing; pG: post-go go trial; pSE: post-stop error go trial; pSEi: post-stop error go trial with increase in reaction time; pSEni: post-stop error go trial without increase in reaction time; pSS: post-stop success go trial; pSSi: post-stop success go trial with increase in reaction time; pSSni: post-stop success go trial without increase in reaction time; preSMA: pre-supplementary motor area; RF: radiofrequency; ROI: region of interest; RT: reaction time; SE: stop error; SFC: superior frontal cortex; SPM: Statistical Parametric Mapping; SS: stop success; SSD: stop-signal delay; SSRT: stop-signal reaction time; SST: stop-signal task; TE: echo time; TR: repetition time; VLPFC: ventrolateral prefrontal cortex.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Li, Cs.R., Zhang, S., Duann, JR. et al. Gender Differences in Cognitive Control: an Extended Investigation of the Stop Signal Task. Brain Imaging and Behavior 3, 262–276 (2009). https://doi.org/10.1007/s11682-009-9068-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-009-9068-1