Abstract
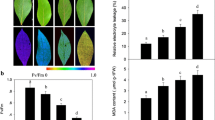
The changes of hydrogen peroxide (H2O2) metabolism and antioxidant enzyme activities in a hybrid poplar (Populus simonii × P. pyramidalis ‘Opera 8277’) in response to mechanical damage (MD) and herbivore wounding (HW) were investigated to determine whether H2O2 could function as the secondary messenger in the signaling of systemic resistance. Results show that H2O2 was generated in wounded leaves through MD and HW treatments and systemically in unwounded leaves around the wounded leaves. The activities of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT) and ascorbate peroxidase (APX) were also enhanced. However, the H2O2 accumulation and antioxidant enzyme activities were inhibited in MD leaves through the pretreatment with DPI (which is a specific inhibitor of NADPH oxidase). The results of this study suggest that H2O2 could be systemically induced by MD and HW treatments, and H2O2 metabolism was closely related to the change in SOD, APX and CAT activities. A high level of antioxidant enzymes could decrease membrane lipid peroxidation levels and effectively induce plant defense responses.
Similar content being viewed by others
References
Blokhina O, Virolainen E, Fagerstedt KV. 2003. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Annals of Botany, 91: 179–194.
Bochkov VN, Kadl A, Huber J, Gruber F, Binder BR, Leitinger N. 2002. Protection role of phospholipids oxidation products in endotoxin-induced tissue damage. Nature, 419: 77–81.
Bolwell GP, Blee KA, Butt VS, Davies DR, Gardner SL, Gerrish C, Minibayeva F, Rowntree EG, Wojtaszek P. 1999. Recent advances in understanding the origin of the apoplastic oxidative burst in plant cells. Free Radical Research, 31: 137–145.
Bostock RM, Karban R, Thaler JS, Weyman PD, Gilchrist D. 2001. Signal interactions in induced resistance to pathogens and insect herbivores. European Journal of Plant Pathology, 107: 103–111.
Bowler C, Fluhr R. 2000. The role of calcium and activated oxygen as signals for controlling cross-tolerance. Trends in Plant Science, 5: 241–246.
Bowler C, Van Montagu M, Inzé D. 1992. Superoxide dismutase and stress tolerance. Annual Review of Plant Physiology and Plant Molecular Biology, 43: 83–116.
Chandru HK, Kim E, Kuk Y, Cho K, Han O. 2003. Kinetics of wound-induced activation of antioxidative enzymes in Oryza Sativa: differential activation at different growth stages. Plant Science, 164: 935–941.
Chang CC, Ball L, Fryer MJ, Baker NR, Karpinski S, Mullineaux PM. 2004. Induction of ascorbate peroxidase 2 expression in wounded Arabidopsis leaves does not involve known wound-signalling pathways but is associated with changes in photosynthesis. Plant Journal, 38: 499–511.
Chen Jianbo, Wang Quanxi, Zhang Jie. 2007. The change of superoxide dismutase’s activity in bean sprout under stress circumstance. Journal of Shanghai Normal University (Natural Sciences), 36: 49–53. (in Chinese with an English abstract)
Costet L, Cordilier S, Dorey S, Baillieul F, Fritig B, Kauffmann S. 1999. Relationship between localized acquired resistance (LAR) and the hypersensitive response (HR): HR is necessary for LAR to occur and salicylic acid is not sufficient to trigger LAR. Molecular Plant Microbe Interactions, 8: 655–662.
Dat JF, Vandenabeele S, Vranová E, Van Montagu M, Inzé D, Van Breuseqem F. 2000. Dual action of the active oxygen species during plant stress responses. Cellular and Molecular Life Sciences, 57: 779–795.
De Vos M, Van Zaanen W, Koornneef A, Korzelius JP, Dicke M, Van Loon LC, Pieterse CMJ. 2006. Herbivore-induced resistance against microbial pathogens in Arabidopsis. Plant Physiology, 142: 352–363.
del Río LA, Corpas FJ, Sandalio LM, Palma JM, Gómez M, Barroso JB. 2002. Reactive oxygen species, antioxidant systems, and nitric oxide in peroxisomes. Journal of Experimental Botany, 53: 1255–1272.
Doke N, Miura Y, Sanchez LM, Park HJ, Noritake T, Yoshioka H Kavakita K. 1996. The oxidative burst protects plants against pathogen attack: mechanism and role as an emergency signal for plant bio-defence. Gene, 179: 45–51.
Fath A, Bethke P, Belligni V, Jones R. 2002. Active oxygen and cell death in cereal aleurone cells. Journal of Experiment Botany, 53: 1273–1282.
Guan LM, Scandalios JG. 2000. Hydrogen peroxide-mediated catalase gene expression in response to wounding. Free Radical Biology and Medicine, 28: 1182–1190.
Lamb CJ, Dixon RA. 1997. The oxidative burst in plant resistance. Annual Review of Plant Physiology and Plant Molecular Biology, 48: 251–275.
Leitner M, Boland W, Mithöfer A. 2005. Direct and indirect defences induced by piercing-sucking and chewing herbivores in Medicago truncatula. New Phytologist, 167: 597–606.
León J, Rojo E, Sánchez-Serrano JJ. 2001. Wound signalling in plants. Journal of Experiment Botany, 52: 1–9.
Liu Jun, Lü Bo, Xu Langlai. 2000. An improved method for the determination of hydrogen peroxide in leaves. Progress in Biochemistry and Biophysics, 27: 548–551. (in Chinese with an English abstract)
Maffei ME, Mithöfer A, Arimura G, Uchtenhagen H, Bossi S, Bertea CM, Cucuzza LS, Novero M, Volpe V, Quadro S, Boland W. 2006. Effects of feeding Spodoptera littoralis on lima bean leaves. III. Membrane depolarization and involvement of hydrogen peroxide. Plant Physiology, 140: 1022–1035.
Mehdy MC. 1994. Active oxygen species in plant defence against pathogens. Plant Physiology, 105: 467–472.
Mithöfer A, Schulze B, Boland W. 2004. Biotic and heavy metal stress response in plants: evidence for common signals. FEBS Lettesr, 566: 1–5.
O’Donel VS, Tew DG, Jones OTG, England PJ. 1993. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochemisty Journal, 290: 41–49.
Orozco-Cárdenas M, Ryan CA. 1999. Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proceedings of National Academy Science of the United States of America, 96: 6553–6557.
Paul ND, Paul Hatcher PE, Taylor JE. 2000. Coping with multiple enemies: an integration of molecular and ecological perspectives. Trends in Plant Science, 5: 220–225.
Sairam, RK, Srivastava GC, Agarwal S, Meena RC. 2005. Differences in antioxidant activity in response to salinity stress in tolerant and susceptible wheat genotypes. Biologia Plantarum, 49: 85–81.
Shen Wenbiao, Xu Langlai, Ye Maobing, Zhang Rongxian. 1996. Study on determination of ASP activity. Plant Physiology Communication, 32: 203–205. (in Chinese with an English abstract)
Somssich IE, Hahlbrock K. 1998. Pathogen defense in plants-a paradigm of biological complexity. Trends in Plant Science, 3: 86–90.
Song Fengming, Ge Xiuchun, Zheng Zhong. 2001. The roles of active oxygen species and lipid peroxidation in the resistance of cotton seedling to fusarium wilt. Acta Phytopathologica Sinica, 31: 110–116. (in Chinese with an English abstract)
Vandenabeele S, Van Der Kelen K, Dat J, Gadjev I, Boonefaes T, Morsa S, Rottiers P, Slooten L, Van Montagu M, Zabeau M, Inzé D, Van Breusegem F. 2003. A comprehensive analysis of hydrogen peroxide-induced gene expression in tobacco. Proceedings of National Academy Science of the United States of America, 23: 16113–16118.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation project: This research is supported by the Key Science Program of the Sate Forestry Administration of China (2006–59), and the National Key Project of Scientific and Technical Supporting Programs Funded by Ministry of Science & Technology of China (2006BAD01A15; 2006BAD24B04).
Biography: AN Yu (1982–), female, Postgraduate in College of Biological Sciences and Biotechnology, Beijing Forestry University, Beijing 100083, P. R. China.
Rights and permissions
About this article
Cite this article
An, Y., Shen, Yb. & Zhang, Zx. Effects of mechanical damage and herbivore wounding on H2O2 metabolism and antioxidant enzyme activities in hybrid poplar leaves. Journal of Forestry Research 20, 156–160 (2009). https://doi.org/10.1007/s11676-009-0027-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-009-0027-x